DP Physics Questionbank

Topic 7: Atomic, nuclear and particle physics
Description
Overview of the essential ideas for this topic
7.1: In the microscopic world energy is discrete.
7.2: Energy can be released in nuclear decays and reactions as a result of the relationship between mass and energy.
7.3: It is believed that all the matter around us is made up of fundamental particles called quarks and leptons. It is known that matter has a hierarchical structure with quarks making up nucleons, nucleons making up nuclei, nuclei and electrons making up atoms and atoms making up molecules. In this hierarchical structure, the smallest scale is seen for quarks and leptons (10–18 m).
Directly related questions
-
16N.2.HL.TZ0.4a:
A particular K meson has a quark structure s. State the charge, strangeness and baryon number for this meson.
- 16N.1.SL.TZ0.24: Photons of energy 2.3eV are incident on a low-pressure vapour. The energy levels of the atoms in...
- 16N.1.SL.TZ0.26: The mass defect for deuterium is 4×10–30 kg. What is the binding energy of deuterium? A....
-
16N.2.HL.TZ0.4b:
The Feynman diagram shows the changes that occur during beta minus (β–) decay.
Label the diagram by inserting the four missing particle symbols and the direction of the arrows for the decay particles.
- 16N.1.SL.TZ0.27: As quarks separate from each other within a hadron, the interaction between them becomes larger....
- 16N.1.HL.TZ0.40: What is the charge on an electron antineutrino and during what process is an electron...
- 16N.1.HL.TZ0.20: Which of the following lists the particles emitted during radioactive decay in order of...
-
16N.1.SL.TZ0.25:
When an alpha particle collides with a nucleus of nitrogen-14 , a nucleus X can be produced together with a proton. What is X?
A.
B.
C.
D.
-
16N.2.SL.TZ0.4a:
A particular K meson has a quark structure s. State the charge on this meson.
- 16N.3.SL.TZ0.3b: The change in foam height can be modelled using ideas from other areas of physics. Identify one...
-
16N.2.SL.TZ0.4c:
Carbon-14 (C-14) is a radioactive isotope which undergoes beta minus (β–) decay to the stable isotope nitrogen-14 (N-14). Energy is released during this decay. Explain why the mass of a C-14 nucleus and the mass of a N-14 nucleus are slightly different even though they have the same nucleon number.
-
16N.3.SL.TZ0.3a:
Determine the time taken for the foam to drop to
(i) half its initial height.
(ii) a quarter of its initial height.
-
17M.1.SL.TZ1.24:
A nucleus of phosphorus (P) decays to a nucleus of silicon (Si) with the emission of particle X and particle Y.
What are X and Y?
- 17M.1.SL.TZ2.24: Atomic spectra are caused when a certain particle makes transitions between energy levels.What is...
- 17M.1.SL.TZ1.15: Two pulses are travelling towards each other. What is a possible pulse shape when the pulses...
-
17M.1.SL.TZ1.27:
What is the energy equivalent to the mass of one proton?
A. 9.38 × (3 × 108)2 × 106 J
B. 9.38 × (3 × 108)2 × 1.6 × 10–19 J
C. J
D. 9.38 × 108 × 1.6 × 10–19 J
-
17M.1.SL.TZ1.25:
What is the definition of the unified atomic mass unit?
A. the mass of a neutral atom of carbon-12
B. The mass of a neutral atom of hydrogen-1
C. the mass of a nucleus of carbon-12
D. The mass of a nucleus of hydrogen-1
-
17M.1.SL.TZ1.26:
In nuclear fission, a nucleus of element X absorbs a neutron (n) to give a nucleus of element Y and a nucleus of element Z.
X + n → Y + Z + 2n
What is and ?
- 17M.1.SL.TZ2.25: The half-life of a radioactive element is 5.0 days. A freshly-prepared sample contains 128 g of...
-
17M.1.SL.TZ2.26:
The binding energy per nucleon of is 6 MeV. What is the energy required to separate the nucleons of this nucleus?
A. 24 MeV
B. 42 MeV
C. 66 MeV
D. 90 MeV
-
17M.1.SL.TZ2.27:
The reaction p+ + n0 → p+ + 0 does not occur because it violates the conservation law of
A. electric charge.
B. baryon number.
C. lepton number.
D. strangeness.
-
17M.1.HL.TZ1.20:
A pure sample of nuclide A and a pure sample of nuclide B have the same activity at time t = 0. Nuclide A has a half-life of T, nuclide B has a half-life of 2T.
What is when t = 4T?
A. 4
B. 2
C.
D.
- 17M.1.HL.TZ2.25: Which of the following leads to a paradigm shift? A. Multi-loop circuits B. Standing waves C....
- 17M.2.SL.TZ1.5a: State the quark structures of a meson and a baryon.
- 17M.2.SL.TZ1.5b.iii: Identify the exchange particle in this decay.
-
17M.2.SL.TZ1.5b.ii:
Draw arrow heads on the lines representing and d in the .
-
17M.2.SL.TZ2.4d:
Rutherford and Royds identified the helium gas in cylinder B by observing its emission spectrum. Outline, with reference to atomic energy levels, how an emission spectrum is formed.
-
17M.1.HL.TZ2.21:
In the nuclear reaction X + Y → Z + W, involving nuclides X, Y, Z and W, energy is released. Which is correct about the masses (M) and the binding energies (BE) of the nuclides?
- 17M.2.SL.TZ1.5c: Outline one benefit of international cooperation in the construction or use of high-energy...
- 17M.2.SL.TZ2.4a: Write down the missing values in the nuclear equation for this decay.
- 17M.2.SL.TZ2.4b: Rutherford and Royds put some pure radium-226 in a small closed cylinder A. Cylinder A is fixed...
-
17M.2.HL.TZ2.5a:
Write down the nuclear equation for this decay.
-
20N.1.SL.TZ0.29:
Four of the energy states for an atom are shown. Transition between any two states is possible.
What is the shortest wavelength of radiation that can be emitted from these four states?
A.
B.
C.
D.
- 20N.1.SL.TZ0.28: What statement about alpha particles, beta particles and gamma radiation is true? A. Gamma...
-
20N.1.SL.TZ0.30:
The Feynman diagram shows some of the changes in a proton–proton collision.
What is the equation for this collision?
A.
B.
C.
D.
-
20N.1.SL.TZ0.27:
Which graph shows the variation of activity with time for a radioactive nuclide?
-
20N.1.HL.TZ0.24:
The mass of nuclear fuel in a nuclear reactor decreases at the rate of every hour. The overall reaction process has an efficiency of . What is the maximum power output of the reactor?
A.
B.
C.
D.
- 20N.2.SL.TZ0.6a(ii): Outline why quantities such as atomic mass and nuclear binding energy are often expressed in...
-
20N.2.SL.TZ0.6c(i):
Write down the proton number of nuclide X.
-
20N.2.SL.TZ0.6a(iii):
Show that the energy released in the reaction is about .
-
20N.2.SL.TZ0.6c(ii):
State the half-life of Sr-94.
- 20N.2.SL.TZ0.6a(i): State what is meant by binding energy of a nucleus.
-
20N.2.SL.TZ0.6c(iii):
Calculate the mass of Sr-94 remaining in the sample after minutes.
- 20N.2.HL.TZ0.6a(i): State what is meant by binding energy of a nucleus.
-
20N.2.HL.TZ0.6c(iii):
Calculate the mass of Sr-94 remaining in the sample after minutes.
- 20N.2.HL.TZ0.6a(ii): Outline why quantities such as atomic mass and nuclear binding energy are often expressed in...
-
20N.2.HL.TZ0.6c(ii):
State the half-life of Sr-94.
-
20N.2.HL.TZ0.6c(i):
Write down the proton number of nuclide X.
-
20N.2.HL.TZ0.6a(iii):
Show that the energy released in the reaction is about .
- 17M.2.HL.TZ2.5c.i: The wall of cylinder A is made from glass. Outline why this glass wall had to be very thin.
- 17N.1.SL.TZ0.23: Which statement about atomic spectra is not true? A. They provide evidence for discrete energy...
- 17N.1.SL.TZ0.24: What gives the total change in nuclear mass and the change in nuclear binding energy as a...
- 17N.1.SL.TZ0.25: The Feynman diagram shows a particle interaction involving a W– boson. Which particles are...
- 17N.2.HL.TZ0.3a.i: State and explain the nature of the particle labelled X.
- 17N.2.SL.TZ0.2b: Distinguish between hadrons and leptons.
-
21M.2.SL.TZ1.5a:
Uranium-238 decays into a nuclide of thorium-234 (Th).
Write down the complete equation for this radioactive decay. -
21M.2.HL.TZ1.7b:
Thallium-206 decays into lead-206 .
Identify the quark changes for this decay.
- 21M.2.HL.TZ1.7d.i: Outline why high temperatures are required for fusion to occur.
- 21M.2.HL.TZ1.7d.ii: Outline, with reference to the graph, why energy is released both in fusion and in fission.
-
21M.2.HL.TZ1.7d.iii:
Uranium-235 is used as a nuclear fuel. The fission of uranium-235 can produce krypton-89 and barium-144.
Determine, in MeV and using the graph, the energy released by this fission.
-
21M.2.SL.TZ1.7b:
When a pi meson π- (du̅) and a proton (uud) collide, a possible outcome is a sigma baryon Σ0 (uds) and a kaon meson Κ0 (ds̅).
Apply three conservation laws to show that this interaction is possible. -
21M.2.HL.TZ2.4a.i:
Write down the equation to represent this decay.
-
21M.2.HL.TZ2.4b:
The neutron number N and the proton number Z are not equal for the nuclide . Explain, with reference to the forces acting within the nucleus, the reason for this.
-
21M.2.HL.TZ2.4c:
Thallium-205 () can also form from successive alpha (α) and beta-minus (β−) decays of an unstable nuclide. The decays follow the sequence α β− β− α. The diagram shows the position of on a chart of neutron number against proton number.
Draw four arrows to show the sequence of changes to N and Z that occur as the forms from the unstable nuclide.
- 21M.1.HL.TZ1.23: Which Feynman diagram describes the annihilation of an electron and its antiparticle?
- 21M.1.HL.TZ1.22: In a hydrogen atom, the sum of the masses of a proton and of an electron is larger than the mass...
- 21M.1.SL.TZ1.24: A simple model of an atom has three energy levels. The differences between adjacent energy levels...
- 21M.1.SL.TZ1.25: What is the relation between the value of the unified atomic mass unit in grams and the value of...
-
21M.1.SL.TZ1.26:
Three particles are produced when the nuclide undergoes beta-plus (β+) decay. What are two of these particles?
A. and
B. and
C. and
D. and
-
21M.1.SL.TZ1.27:
A particle reaction is
.
Which conservation law is violated by the reaction?
A. Baryon number
B. Charge
C. Lepton number
D. Momentum
- 21M.1.SL.TZ2.28: Consider the Feynman diagram below. What is the exchange particle X? A. Lepton B. Gluon C....
-
21M.1.SL.TZ2.26:
The diagram below shows four energy levels for the atoms of a gas. The diagram is drawn to scale. The wavelengths of the photons emitted by the energy transitions between levels are shown.
What are the wavelengths of spectral lines, emitted by the gas, in order of decreasing frequency?
A.
B.
C.
D.
-
21M.1.SL.TZ2.25:
When a high-energy -particle collides with a beryllium-9 () nucleus, a nucleus of carbon may be produced. What are the products of this reaction?
- 21M.1.SL.TZ2.27: A kaon is made up of two quarks. What is the particle classification of a kaon? A. Exchange...
-
21M.1.HL.TZ2.20:
A sample of a pure radioactive nuclide initially contains atoms. The initial activity of the sample is .
A second sample of the same nuclide initially contains atoms.
What is the activity of the second sample after three half lives?
A.
B.
C.
D.
-
21M.1.HL.TZ2.22:
During the nuclear fission of nucleus X into nucleus Y and nucleus Z, energy is released. The binding energies per nucleon of X, Y and Z are , and respectively. What is true about the binding energy per nucleon of X, Y and Z?
A. > and >B. = and =
C. > and >
D. = +
-
21M.2.HL.TZ1.7a:
Uranium-238 decays into a nuclide of thorium-234 (Th).
Write down the complete equation for this radioactive decay. -
21M.2.SL.TZ1.5b:
Thallium-206 decays into lead-206 .
Identify the quark changes for this decay.
- 21M.2.SL.TZ1.5c.i: Outline why high temperatures are required for fusion to occur
- 21M.2.SL.TZ1.5c.ii: Outline, with reference to the graph, why energy is released both in fusion and in fission.
-
21M.2.SL.TZ2.4a:
Write down the equation to represent this decay.
-
21M.2.SL.TZ1.5c.iii:
Uranium-235 () is used as a nuclear fuel. The fission of uranium-235 can produce krypton-89 and barium-144.
Determine, in MeV and using the graph, the energy released by this fission.
-
21M.2.SL.TZ2.4b:
The neutron number N and the proton number Z are not equal for the nuclide . Explain, with reference to the forces acting within the nucleus, the reason for this.
-
21M.2.SL.TZ2.4c:
Thallium-205 () can also form from successive alpha (α) and beta-minus (β−) decays of an unstable nuclide. The decays follow the sequence α β− β− α. The diagram shows the position of on a chart of neutron number against proton number.
Draw four arrows to show the sequence of changes to N and Z that occur as the forms from the unstable nuclide.
-
21N.2.SL.TZ0.5b.ii:
The plutonium nucleus is at rest when it decays.
Calculate the ratio .
-
18M.1.SL.TZ1.24:
Which Feynman diagram shows beta-plus (β+) decay?
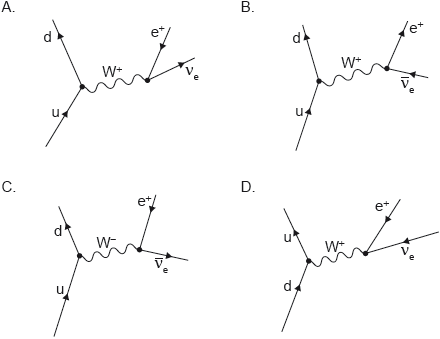
-
18M.1.SL.TZ1.26:
Two pure samples of radioactive nuclides X and Y have the same initial number of atoms. The half-life of X is .
After a time equal to 4 half-lives of X the ratio is .
What is the half-life of Y?
A.
B.
C.
D.
- 18M.1.SL.TZ1.27: The energy-level diagram for an atom that has four energy states is shown. ...
- 18M.1.HL.TZ1.21: What is correct about the Higgs Boson? A. It was predicted before it was observed. B. ...
-
18M.1.SL.TZ1.25:
The average binding energy per nucleon of the nucleus is 7.5 MeV. What is the total energy required to separate the nucleons of one nucleus of ?
A. 53 MeV
B. 60 MeV
C. 113 MeV
D. 173 MeV
-
18M.2.SL.TZ1.6a:
Identify the missing information for this decay.
-
18M.2.SL.TZ1.6b.i:
On the graph, sketch how the number of boron nuclei in the sample varies with time.
-
18M.2.SL.TZ1.6b.ii:
After 4.3 × 106 years,
Show that the half-life of beryllium-10 is 1.4 × 106 years.
-
18M.2.SL.TZ1.6b.iii:
Beryllium-10 is used to investigate ice samples from Antarctica. A sample of ice initially contains 7.6 × 1011 atoms of beryllium-10. State the number of remaining beryllium-10 nuclei in the sample after 2.8 × 106 years.
-
18M.3.HL.TZ1.6a.i:
write down the momentum of the neutrino.
- 18M.1.HL.TZ2.20: Identify the conservation law violated in the proposed reaction. ...
-
18M.1.SL.TZ2.25:
Element X decays through a series of alpha (α) and beta minus (β–) emissions. Which series of emissions results in an isotope of X?
A. 1α and 2β–
B. 1α and 4β–
C. 2α and 2β–
D. 2α and 3β–
- 18M.1.SL.TZ2.27: Three of the fundamental forces between particles are I. strong nuclear ...
- 18M.1.SL.TZ2.24: A detector, placed close to a radioactive source, detects an activity of 260 Bq. The...
- 18M.1.SL.TZ2.26: A graph of the variation of average binding energy per nucleon with nucleon number has a maximum....
-
18M.2.HL.TZ2.9d.ii:
Suggest why the β– decay is followed by the emission of a gamma ray photon.
-
18M.2.SL.TZ2.6c.ii:
Identify particle V.
-
18M.2.SL.TZ2.6a:
Rutherford constructed a model of the atom based on the results of the alpha particle scattering experiment. Describe this model.
-
18M.2.SL.TZ2.6b.i:
State what is meant by the binding energy of a nucleus.
-
18M.2.SL.TZ2.6b.ii:
Show that the energy released in the β– decay of rhodium is about 3 MeV.
-
18M.2.SL.TZ2.6c.i:
Draw a labelled arrow to complete the Feynman diagram.
-
18M.2.HL.TZ1.6a:
Identify the missing information for this decay.
- 21N.1.SL.TZ0.26: A proton collides with an electron. What are the possible products of the collision? A. Two...
-
21N.1.SL.TZ0.24:
A pure sample of radioactive nuclide decays into a stable nuclide .
What is after two half-lives?
A. 1B. 2
C. 3
D. 4
-
21N.1.SL.TZ0.25:
The mass of a nucleus of iron-56 () is M.
What is the mass defect of the nucleus of iron-56?
A. M − 26mp − 56mn
B. 26mp + 30mn − M
C. M − 26mp − 56mn − 26me
D. 26mp + 30mn + 26me − M
- 21N.1.SL.TZ0.27: The Higgs boson was discovered in the Large Hadron Collider at CERN. Which statements are correct...
- 21N.1.HL.TZ0.22: The Feynman diagram shows an interaction between a proton and an electron. What is the charge...
- 21N.1.HL.TZ0.20: A detector measures the count rate from a sample of a radioactive nuclide. The graph shows the...
- 21N.2.SL.TZ0.5a.i: State what is meant by the binding energy of a nucleus.
-
21N.2.SL.TZ0.5a.ii:
Draw, on the axes, a graph to show the variation with nucleon number of the binding energy per nucleon, . Numbers are not required on the vertical axis.
-
21N.2.SL.TZ0.5a.iii:
Identify, with a cross, on the graph in (a)(ii), the region of greatest stability.
-
21N.2.SL.TZ0.5b.i:
Show that the energy released in this decay is about 6 MeV.
-
21N.2.HL.TZ0.4a.iii:
Identify, with a cross, on the graph in (a)(ii), the region of greatest stability.
- 21N.2.HL.TZ0.4a.iv: Some unstable nuclei have many more neutrons than protons. Suggest the likely decay for these...
-
21N.2.HL.TZ0.4b.ii:
The plutonium nucleus is at rest when it decays.
Calculate the ratio .
-
21N.2.HL.TZ0.4a.ii:
Draw, on the axes, a graph to show the variation with nucleon number of the binding energy per nucleon, . Numbers are not required on the vertical axis.
- 21N.2.HL.TZ0.4a.i: State what is meant by the binding energy of a nucleus.
-
21N.2.HL.TZ0.4b.i:
Show that the energy released in this decay is about 6 MeV.
-
18M.2.HL.TZ1.6b.i:
On the graph, sketch how the number of boron nuclei in the sample varies with time.
-
18M.2.HL.TZ1.6b.ii:
After 4.3 × 106 years,
Show that the half-life of beryllium-10 is 1.4 × 106 years.
- 18N.1.SL.TZ0.25: The graph shows the variation of the number of neutrons N with the atomic number Z for stable...
- 18N.1.HL.TZ0.38: Which is the correct Feynman diagram for pair annihilation and pair production?
-
18N.1.SL.TZ0.26:
Copper () decays to nickel (). What are the particles emitted and the particle that mediates the interaction?
- 18N.1.HL.TZ0.20: In the Rutherford-Geiger-Marsden scattering experiment it was observed that a small percentage of...
-
18N.1.HL.TZ0.22:
The following decay is observed.
μ− → e− + vμ + X
What is particle X?
A. γ
B. e
C. Z0
D. ve
-
18N.1.SL.TZ0.27:
The following interaction is proposed between a proton and a pion.
p+ + – → K– + +
The quark content of the – is ūd and the quark content of the K– is ūs.
Three conservation rules are considered
I. baryon number
II. charge
III. strangeness.
Which conservation rules are violated in this interaction?
A. I and II only
B. I and III only
C. II and III only
D. I, II and III
-
18N.2.SL.TZ0.5b:
Identify, with an arrow labelled B on the diagram, the transition in the hydrogen spectrum that gives rise to the photon with the energy in (a).
-
18N.2.SL.TZ0.5c:
Explain your answer to (b).
-
18N.2.HL.TZ0.5a.iii:
Explain your answer to (a)(ii).
- 18N.1.SL.TZ0.24: The graph shows the variation with time of the activity of a pure sample of a radioactive...
-
18N.2.SL.TZ0.5a:
Determine the energy of a photon of blue light (435nm) emitted in the hydrogen spectrum.
-
18N.2.HL.TZ0.5a.i:
Determine the energy of a photon of blue light (435nm) emitted in the hydrogen spectrum.
-
18N.2.HL.TZ0.5a.ii:
Identify, with an arrow labelled B on the diagram, the transition in the hydrogen spectrum that gives rise to the photon with the energy in (a)(i).
-
18N.2.HL.TZ0.6c:
undergoes beta-minus (β–) decay. Explain why the energy gained by the emitted beta particles in this decay is not the same for every beta particle.
- 22M.1.SL.TZ1.26: The background count in a laboratory is 20 counts per second. The initial observed count rate of...
- 22M.1.SL.TZ1.24: Some transitions between the energy states of a particular atom are shown. Energy transition...
- 22M.1.SL.TZ1.25: Three statements about radioactive decay are: I. The rate of decay is exponential.II. It is...
-
22M.1.SL.TZ1.27:
undergoes an alpha decay, followed by a beta-minus decay. What is the number of protons and neutrons in the resulting nuclide?
-
22M.1.HL.TZ1.25:
A pure sample of iodine-131 decays into xenon with a half-life of 8 days.
What is after 24 days?
A.
B.
C.
D.
-
22M.1.HL.TZ1.26:
The diagram shows atomic transitions E1, E2 and E3 when a particular atom changes its energy state. The wavelengths of the photons that correspond to these transitions are , and .
What is correct for these wavelengths?
A.
B.
C.
D.
-
22M.1.HL.TZ1.27:
Carbon (C-12) and hydrogen (H-1) undergo nuclear fusion to form nitrogen.
photon
What is the number of neutrons and number of nucleons in the nitrogen nuclide?
- 22M.2.SL.TZ1.5c: The K+ meson can decay as K+ → μ+ + vμ. State and explain the interaction that is responsible...
-
22M.2.SL.TZ1.5a:
Describe the quark structure of a baryon.
- 22M.2.SL.TZ1.5b: The Feynman diagram shows a possible decay of the K+ meson. Identify the interactions that are...
-
22M.2.HL.TZ1.9a:
Write down the equation for this decay.
- 22M.1.SL.TZ2.26: The energy levels of an atom are shown. How many photons of energy greater than 1.9 eV can be...
- 22M.1.SL.TZ2.25: Three statements about electrons are: I. Electrons interact through virtual photons.II. ...
- 22M.1.SL.TZ2.28: The age of the Earth is about 4.5 × 109 years. What area of physics provides experimental...
- 22M.1.HL.TZ2.22: White light is emitted from a hot filament. The light passes through hydrogen gas at low pressure...
-
22M.1.SL.TZ2.27:
What statement is not true about radioactive decay?
A. The percentage of radioactive nuclei of an isotope in a sample of that isotope after 7 half-lives is smaller than 1 %.B. The half-life of a radioactive isotope is the time taken for half the nuclei in a sample of that isotope to decay.
C. The whole-life of a radioactive isotope is the time taken for all the nuclei in a sample of that isotope to decay.
D. The half-life of radioactive isotopes range between extremely short intervals to thousands of millions of years.
-
22M.1.HL.TZ2.24:
A neutron is absorbed by a nucleus of uranium-235. One possible outcome is the production of two nuclides, barium-144 and krypton-89.
How many neutrons are released in this reaction?
A. 0
B. 1
C. 2
D. 3
-
22M.1.HL.TZ2.25:
A radioactive nuclide X decays into a nuclide Y. The graph shows the variation with time of the activity A of X. X and Y have the same nucleon number.
What is true about nuclide X?
A. alpha (α) emitter with a half-life of t
B. alpha (α) emitter with a half-life of 2t
C. beta-minus (β−) emitter with a half-life of t
D. beta-minus (β−) emitter with a half-life of 2t
- 22M.2.SL.TZ2.5a: Outline how the count rate was corrected for background radiation.
- 22M.2.SL.TZ2.5b: When a single piece of thin copper foil is placed between the source and detector, the count rate...
- 22M.2.SL.TZ2.5c: Further results were obtained in this experiment with copper and lead absorbers. Comment on...
-
22M.2.SL.TZ2.5d:
Another radioactive source consists of a nuclide of caesium that decays to barium .
Write down the reaction for this decay.
- 19M.2.SL.TZ2.6ci: Identify, for particle Y, the charge.
- 19M.2.SL.TZ2.6bi: Determine, in MeV, the energy released.
- 19M.2.SL.TZ2.6cii: Identify, for particle Y, the strangeness.
-
19M.2.SL.TZ2.6a:
Identify particle X.
- 19M.2.SL.TZ2.6bii: Suggest why, for the fusion reaction above to take place, the temperature of deuterium must be...
-
19M.2.SL.TZ1.2a.iii:
Energy is transferred to a hadron in an attempt to separate its quarks. Describe the implications of quark confinement for this situation.
- 19M.2.SL.TZ1.2b: The Standard Model was accepted by many scientists before the observation of the Higgs boson was...
-
19M.2.SL.TZ1.2a.ii:
Sketch the Feynman diagram that represents this reaction. The diagram has been started for you.
-
19M.1.HL.TZ2.34:
The meson contains an up () quark. What is the quark structure of the meson?
A.
B.
C.
D.
-
19M.2.SL.TZ1.2a.i:
Write down the nuclear equation that represents this reaction.
-
19M.1.SL.TZ1.28:
Which of the following atomic energy level transitions corresponds to photons of the shortest wavelength?
-
19M.1.SL.TZ2.26:
Three conservation laws in nuclear reactions are
I. conservation of charge
II. conservation of baryon number
III. conservation of lepton number.
The reaction
is proposed.
Which conservation laws are violated in the proposed reaction?
A. I and II only
B. I and III only
C. II and III only
D. I, II and III
- 19M.1.SL.TZ2.27: Which Feynman diagram shows the emission of a photon by a charged antiparticle?
-
19M.1.SL.TZ2.25:
The positions of stable nuclei are plotted by neutron number n and proton number p. The graph indicates a dotted line for which n = p. Which graph shows the line of stable nuclides and the shaded region where unstable nuclei emit beta minus (β-) particles?
-
19M.1.SL.TZ2.24:
A radioactive nuclide with atomic number Z undergoes a process of beta-plus (β+) decay. What is the atomic number for the nuclide produced and what is another particle emitted during the decay?
-
19M.1.HL.TZ1.22:
The diagram shows the emission spectrum of an atom.
Which of the following atomic energy level models can produce this spectrum?
-
19M.1.SL.TZ1.27:
The rest mass of the helium isotope is m.
Which expression gives the binding energy per nucleon for ?
A.
B.
C.
D.
-
19M.1.SL.TZ1.26:
Which property of a nuclide does not change as a result of beta decay?
A. Nucleon number
B. Neutron number
C. Proton number
D. Charge
-
19M.1.HL.TZ1.23:
The carbon isotope C is radioactive. It decays according to the equation
C → N + X + Y
What are X and Y?
-
19N.1.HL.TZ0.19:
Nuclide X can decay by two routes. In Route 1 alpha (α) decay is followed by beta-minus (β–) decay. In Route 2 β– decay is followed by α decay. P and R are the intermediate products and Q and S are the final products.
Which statement is correct?
A. Q and S are different isotopes of the same element.
B. The mass numbers of X and R are the same.
C. The atomic numbers of P and R are the same.
D. X and R are different isotopes of the same element.
- 19N.1.SL.TZ0.27: What is correct about the nature and range of the strong interaction between nuclear...
- 19N.1.SL.TZ0.24: The energy levels for an atom are shown to scale. A photon of wavelength λ is emitted because of...
-
19N.1.SL.TZ0.26:
X is a radioactive nuclide that decays to a stable nuclide. The activity of X falls to th of its original value in 32 s.
What is the half-life of X?A. 2 s
B. 4 s
C. 8 s
D. 16 s
- 19N.1.SL.TZ0.25: A proton, an electron and an alpha particle are at rest. Which particle has the smallest...
-
19N.1.HL.TZ0.21:
Gamma () radiation
A. is deflected by a magnetic field.
B. affects a photographic plate.
C. originates in the electron cloud outside a nucleus.
D. is deflected by an electric field.
- 19N.2.SL.TZ0.7a: Radioactive decay is said to be “random” and “spontaneous”. Outline what is meant by each of...
-
19N.2.SL.TZ0.7b(i):
Calculate the binding energy per nucleon for uranium-238.
-
19N.2.SL.TZ0.7b(ii):
Calculate the ratio .
Sub sections and their related questions
7.1 – Discrete energy and radioactivity
- 16N.1.SL.TZ0.24: Photons of energy 2.3eV are incident on a low-pressure vapour. The energy levels of the atoms in...
- 16N.1.HL.TZ0.20: Which of the following lists the particles emitted during radioactive decay in order of...
- 16N.1.HL.TZ0.40: What is the charge on an electron antineutrino and during what process is an electron...
-
16N.3.SL.TZ0.3a:
Determine the time taken for the foam to drop to
(i) half its initial height.
(ii) a quarter of its initial height.
- 16N.3.SL.TZ0.3b: The change in foam height can be modelled using ideas from other areas of physics. Identify one...
- 17M.1.SL.TZ1.15: Two pulses are travelling towards each other. What is a possible pulse shape when the pulses...
-
17M.1.SL.TZ1.24:
A nucleus of phosphorus (P) decays to a nucleus of silicon (Si) with the emission of particle X and particle Y.
What are X and Y?
-
17M.1.HL.TZ1.20:
A pure sample of nuclide A and a pure sample of nuclide B have the same activity at time t = 0. Nuclide A has a half-life of T, nuclide B has a half-life of 2T.
What is when t = 4T?
A. 4
B. 2
C.
D.
- 17M.1.SL.TZ2.24: Atomic spectra are caused when a certain particle makes transitions between energy levels.What is...
- 17M.1.SL.TZ2.25: The half-life of a radioactive element is 5.0 days. A freshly-prepared sample contains 128 g of...
- 17M.1.HL.TZ2.25: Which of the following leads to a paradigm shift? A. Multi-loop circuits B. Standing waves C....
- 17M.2.SL.TZ2.4a: Write down the missing values in the nuclear equation for this decay.
- 17M.2.SL.TZ2.4b: Rutherford and Royds put some pure radium-226 in a small closed cylinder A. Cylinder A is fixed...
-
17M.2.SL.TZ2.4d:
Rutherford and Royds identified the helium gas in cylinder B by observing its emission spectrum. Outline, with reference to atomic energy levels, how an emission spectrum is formed.
-
17M.2.HL.TZ2.5a:
Write down the nuclear equation for this decay.
- 17M.2.HL.TZ2.5c.i: The wall of cylinder A is made from glass. Outline why this glass wall had to be very thin.
- 17N.1.SL.TZ0.23: Which statement about atomic spectra is not true? A. They provide evidence for discrete energy...
- 17N.2.HL.TZ0.3a.i: State and explain the nature of the particle labelled X.
-
18M.1.SL.TZ1.26:
Two pure samples of radioactive nuclides X and Y have the same initial number of atoms. The half-life of X is .
After a time equal to 4 half-lives of X the ratio is .
What is the half-life of Y?
A.
B.
C.
D.
- 18M.1.SL.TZ1.27: The energy-level diagram for an atom that has four energy states is shown. ...
-
18M.2.SL.TZ1.6a:
Identify the missing information for this decay.
-
18M.2.SL.TZ1.6b.i:
On the graph, sketch how the number of boron nuclei in the sample varies with time.
-
18M.2.SL.TZ1.6b.ii:
After 4.3 × 106 years,
Show that the half-life of beryllium-10 is 1.4 × 106 years.
-
18M.2.SL.TZ1.6b.iii:
Beryllium-10 is used to investigate ice samples from Antarctica. A sample of ice initially contains 7.6 × 1011 atoms of beryllium-10. State the number of remaining beryllium-10 nuclei in the sample after 2.8 × 106 years.
- 18M.1.SL.TZ2.24: A detector, placed close to a radioactive source, detects an activity of 260 Bq. The...
-
18M.1.SL.TZ2.25:
Element X decays through a series of alpha (α) and beta minus (β–) emissions. Which series of emissions results in an isotope of X?
A. 1α and 2β–
B. 1α and 4β–
C. 2α and 2β–
D. 2α and 3β–
- 18M.1.SL.TZ2.27: Three of the fundamental forces between particles are I. strong nuclear ...
-
18M.3.HL.TZ1.6a.i:
write down the momentum of the neutrino.
-
18M.2.HL.TZ2.9d.ii:
Suggest why the β– decay is followed by the emission of a gamma ray photon.
-
18M.2.HL.TZ1.6a:
Identify the missing information for this decay.
-
18M.2.HL.TZ1.6b.i:
On the graph, sketch how the number of boron nuclei in the sample varies with time.
-
18M.2.HL.TZ1.6b.ii:
After 4.3 × 106 years,
Show that the half-life of beryllium-10 is 1.4 × 106 years.
- 18N.1.SL.TZ0.24: The graph shows the variation with time of the activity of a pure sample of a radioactive...
-
18N.1.SL.TZ0.26:
Copper () decays to nickel (). What are the particles emitted and the particle that mediates the interaction?
-
18N.2.SL.TZ0.5a:
Determine the energy of a photon of blue light (435nm) emitted in the hydrogen spectrum.
-
18N.2.SL.TZ0.5b:
Identify, with an arrow labelled B on the diagram, the transition in the hydrogen spectrum that gives rise to the photon with the energy in (a).
-
18N.2.SL.TZ0.5c:
Explain your answer to (b).
-
18N.2.HL.TZ0.5a.i:
Determine the energy of a photon of blue light (435nm) emitted in the hydrogen spectrum.
-
18N.2.HL.TZ0.5a.ii:
Identify, with an arrow labelled B on the diagram, the transition in the hydrogen spectrum that gives rise to the photon with the energy in (a)(i).
-
18N.2.HL.TZ0.5a.iii:
Explain your answer to (a)(ii).
-
18N.2.HL.TZ0.6c:
undergoes beta-minus (β–) decay. Explain why the energy gained by the emitted beta particles in this decay is not the same for every beta particle.
-
19M.1.SL.TZ1.28:
Which of the following atomic energy level transitions corresponds to photons of the shortest wavelength?
-
19M.2.SL.TZ1.2a.i:
Write down the nuclear equation that represents this reaction.
-
19M.1.SL.TZ2.24:
A radioactive nuclide with atomic number Z undergoes a process of beta-plus (β+) decay. What is the atomic number for the nuclide produced and what is another particle emitted during the decay?
-
19M.1.HL.TZ1.22:
The diagram shows the emission spectrum of an atom.
Which of the following atomic energy level models can produce this spectrum?
-
19M.1.HL.TZ1.23:
The carbon isotope C is radioactive. It decays according to the equation
C → N + X + Y
What are X and Y?
- 19N.1.SL.TZ0.24: The energy levels for an atom are shown to scale. A photon of wavelength λ is emitted because of...
- 19N.1.SL.TZ0.25: A proton, an electron and an alpha particle are at rest. Which particle has the smallest...
-
19N.1.SL.TZ0.26:
X is a radioactive nuclide that decays to a stable nuclide. The activity of X falls to th of its original value in 32 s.
What is the half-life of X?A. 2 s
B. 4 s
C. 8 s
D. 16 s
-
19N.1.HL.TZ0.19:
Nuclide X can decay by two routes. In Route 1 alpha (α) decay is followed by beta-minus (β–) decay. In Route 2 β– decay is followed by α decay. P and R are the intermediate products and Q and S are the final products.
Which statement is correct?
A. Q and S are different isotopes of the same element.
B. The mass numbers of X and R are the same.
C. The atomic numbers of P and R are the same.
D. X and R are different isotopes of the same element.
-
19N.1.HL.TZ0.21:
Gamma () radiation
A. is deflected by a magnetic field.
B. affects a photographic plate.
C. originates in the electron cloud outside a nucleus.
D. is deflected by an electric field.
- 19N.2.SL.TZ0.7a: Radioactive decay is said to be “random” and “spontaneous”. Outline what is meant by each of...
-
20N.1.SL.TZ0.27:
Which graph shows the variation of activity with time for a radioactive nuclide?
- 20N.1.SL.TZ0.28: What statement about alpha particles, beta particles and gamma radiation is true? A. Gamma...
-
20N.1.SL.TZ0.29:
Four of the energy states for an atom are shown. Transition between any two states is possible.
What is the shortest wavelength of radiation that can be emitted from these four states?
A.
B.
C.
D.
-
20N.2.SL.TZ0.6c(i):
Write down the proton number of nuclide X.
-
20N.2.SL.TZ0.6c(ii):
State the half-life of Sr-94.
-
20N.2.SL.TZ0.6c(iii):
Calculate the mass of Sr-94 remaining in the sample after minutes.
-
20N.2.HL.TZ0.6c(i):
Write down the proton number of nuclide X.
-
20N.2.HL.TZ0.6c(ii):
State the half-life of Sr-94.
-
20N.2.HL.TZ0.6c(iii):
Calculate the mass of Sr-94 remaining in the sample after minutes.
-
21M.2.SL.TZ1.5a:
Uranium-238 decays into a nuclide of thorium-234 (Th).
Write down the complete equation for this radioactive decay. -
21M.2.HL.TZ2.4a.i:
Write down the equation to represent this decay.
-
21M.2.HL.TZ2.4c:
Thallium-205 () can also form from successive alpha (α) and beta-minus (β−) decays of an unstable nuclide. The decays follow the sequence α β− β− α. The diagram shows the position of on a chart of neutron number against proton number.
Draw four arrows to show the sequence of changes to N and Z that occur as the forms from the unstable nuclide.
- 21M.1.SL.TZ1.24: A simple model of an atom has three energy levels. The differences between adjacent energy levels...
-
21M.1.SL.TZ1.26:
Three particles are produced when the nuclide undergoes beta-plus (β+) decay. What are two of these particles?
A. and
B. and
C. and
D. and
-
21M.1.SL.TZ2.26:
The diagram below shows four energy levels for the atoms of a gas. The diagram is drawn to scale. The wavelengths of the photons emitted by the energy transitions between levels are shown.
What are the wavelengths of spectral lines, emitted by the gas, in order of decreasing frequency?
A.
B.
C.
D.
-
21M.1.SL.TZ2.25:
When a high-energy -particle collides with a beryllium-9 () nucleus, a nucleus of carbon may be produced. What are the products of this reaction?
-
21M.1.HL.TZ2.20:
A sample of a pure radioactive nuclide initially contains atoms. The initial activity of the sample is .
A second sample of the same nuclide initially contains atoms.
What is the activity of the second sample after three half lives?
A.
B.
C.
D.
-
21M.2.HL.TZ1.7a:
Uranium-238 decays into a nuclide of thorium-234 (Th).
Write down the complete equation for this radioactive decay. -
21M.2.SL.TZ2.4a:
Write down the equation to represent this decay.
-
21M.2.SL.TZ2.4c:
Thallium-205 () can also form from successive alpha (α) and beta-minus (β−) decays of an unstable nuclide. The decays follow the sequence α β− β− α. The diagram shows the position of on a chart of neutron number against proton number.
Draw four arrows to show the sequence of changes to N and Z that occur as the forms from the unstable nuclide.
-
21N.1.SL.TZ0.24:
A pure sample of radioactive nuclide decays into a stable nuclide .
What is after two half-lives?
A. 1B. 2
C. 3
D. 4
- 21N.1.HL.TZ0.20: A detector measures the count rate from a sample of a radioactive nuclide. The graph shows the...
- 21N.2.HL.TZ0.4a.iv: Some unstable nuclei have many more neutrons than protons. Suggest the likely decay for these...
- 22M.1.SL.TZ2.26: The energy levels of an atom are shown. How many photons of energy greater than 1.9 eV can be...
-
22M.1.SL.TZ2.27:
What statement is not true about radioactive decay?
A. The percentage of radioactive nuclei of an isotope in a sample of that isotope after 7 half-lives is smaller than 1 %.B. The half-life of a radioactive isotope is the time taken for half the nuclei in a sample of that isotope to decay.
C. The whole-life of a radioactive isotope is the time taken for all the nuclei in a sample of that isotope to decay.
D. The half-life of radioactive isotopes range between extremely short intervals to thousands of millions of years.
- 22M.1.SL.TZ2.28: The age of the Earth is about 4.5 × 109 years. What area of physics provides experimental...
- 22M.1.HL.TZ2.22: White light is emitted from a hot filament. The light passes through hydrogen gas at low pressure...
-
22M.1.HL.TZ2.25:
A radioactive nuclide X decays into a nuclide Y. The graph shows the variation with time of the activity A of X. X and Y have the same nucleon number.
What is true about nuclide X?
A. alpha (α) emitter with a half-life of t
B. alpha (α) emitter with a half-life of 2t
C. beta-minus (β−) emitter with a half-life of t
D. beta-minus (β−) emitter with a half-life of 2t
- 22M.2.SL.TZ2.5a: Outline how the count rate was corrected for background radiation.
- 22M.2.SL.TZ2.5b: When a single piece of thin copper foil is placed between the source and detector, the count rate...
- 22M.2.SL.TZ2.5c: Further results were obtained in this experiment with copper and lead absorbers. Comment on...
-
22M.2.SL.TZ2.5d:
Another radioactive source consists of a nuclide of caesium that decays to barium .
Write down the reaction for this decay.
- 22M.1.SL.TZ1.24: Some transitions between the energy states of a particular atom are shown. Energy transition...
- 22M.1.SL.TZ1.25: Three statements about radioactive decay are: I. The rate of decay is exponential.II. It is...
- 22M.1.SL.TZ1.26: The background count in a laboratory is 20 counts per second. The initial observed count rate of...
-
22M.1.SL.TZ1.27:
undergoes an alpha decay, followed by a beta-minus decay. What is the number of protons and neutrons in the resulting nuclide?
-
22M.1.HL.TZ1.25:
A pure sample of iodine-131 decays into xenon with a half-life of 8 days.
What is after 24 days?
A.
B.
C.
D.
-
22M.1.HL.TZ1.26:
The diagram shows atomic transitions E1, E2 and E3 when a particular atom changes its energy state. The wavelengths of the photons that correspond to these transitions are , and .
What is correct for these wavelengths?
A.
B.
C.
D.
-
22M.1.HL.TZ1.27:
Carbon (C-12) and hydrogen (H-1) undergo nuclear fusion to form nitrogen.
photon
What is the number of neutrons and number of nucleons in the nitrogen nuclide?
-
22M.2.HL.TZ1.9a:
Write down the equation for this decay.
7.2 – Nuclear reactions
-
16N.1.SL.TZ0.25:
When an alpha particle collides with a nucleus of nitrogen-14 , a nucleus X can be produced together with a proton. What is X?
A.
B.
C.
D.
- 16N.1.SL.TZ0.26: The mass defect for deuterium is 4×10–30 kg. What is the binding energy of deuterium? A....
-
16N.2.SL.TZ0.4c:
Carbon-14 (C-14) is a radioactive isotope which undergoes beta minus (β–) decay to the stable isotope nitrogen-14 (N-14). Energy is released during this decay. Explain why the mass of a C-14 nucleus and the mass of a N-14 nucleus are slightly different even though they have the same nucleon number.
- 17M.1.SL.TZ1.15: Two pulses are travelling towards each other. What is a possible pulse shape when the pulses...
-
17M.1.SL.TZ1.25:
What is the definition of the unified atomic mass unit?
A. the mass of a neutral atom of carbon-12
B. The mass of a neutral atom of hydrogen-1
C. the mass of a nucleus of carbon-12
D. The mass of a nucleus of hydrogen-1
-
17M.1.SL.TZ1.26:
In nuclear fission, a nucleus of element X absorbs a neutron (n) to give a nucleus of element Y and a nucleus of element Z.
X + n → Y + Z + 2n
What is and ?
-
17M.1.SL.TZ1.27:
What is the energy equivalent to the mass of one proton?
A. 9.38 × (3 × 108)2 × 106 J
B. 9.38 × (3 × 108)2 × 1.6 × 10–19 J
C. J
D. 9.38 × 108 × 1.6 × 10–19 J
-
17M.1.SL.TZ2.26:
The binding energy per nucleon of is 6 MeV. What is the energy required to separate the nucleons of this nucleus?
A. 24 MeV
B. 42 MeV
C. 66 MeV
D. 90 MeV
-
17M.1.HL.TZ2.21:
In the nuclear reaction X + Y → Z + W, involving nuclides X, Y, Z and W, energy is released. Which is correct about the masses (M) and the binding energies (BE) of the nuclides?
- 17N.1.SL.TZ0.24: What gives the total change in nuclear mass and the change in nuclear binding energy as a...
-
18M.1.SL.TZ1.25:
The average binding energy per nucleon of the nucleus is 7.5 MeV. What is the total energy required to separate the nucleons of one nucleus of ?
A. 53 MeV
B. 60 MeV
C. 113 MeV
D. 173 MeV
- 18M.1.SL.TZ2.26: A graph of the variation of average binding energy per nucleon with nucleon number has a maximum....
-
18M.2.SL.TZ2.6b.i:
State what is meant by the binding energy of a nucleus.
-
18M.2.SL.TZ2.6b.ii:
Show that the energy released in the β– decay of rhodium is about 3 MeV.
- 18N.1.SL.TZ0.25: The graph shows the variation of the number of neutrons N with the atomic number Z for stable...
-
19M.2.SL.TZ2.6a:
Identify particle X.
- 19M.2.SL.TZ2.6bi: Determine, in MeV, the energy released.
- 19M.2.SL.TZ2.6bii: Suggest why, for the fusion reaction above to take place, the temperature of deuterium must be...
- 19M.2.SL.TZ2.6ci: Identify, for particle Y, the charge.
- 19M.2.SL.TZ2.6cii: Identify, for particle Y, the strangeness.
-
19M.1.SL.TZ1.26:
Which property of a nuclide does not change as a result of beta decay?
A. Nucleon number
B. Neutron number
C. Proton number
D. Charge
-
19M.1.SL.TZ1.27:
The rest mass of the helium isotope is m.
Which expression gives the binding energy per nucleon for ?
A.
B.
C.
D.
-
19M.1.SL.TZ2.25:
The positions of stable nuclei are plotted by neutron number n and proton number p. The graph indicates a dotted line for which n = p. Which graph shows the line of stable nuclides and the shaded region where unstable nuclei emit beta minus (β-) particles?
-
19N.2.SL.TZ0.7b(i):
Calculate the binding energy per nucleon for uranium-238.
-
19N.2.SL.TZ0.7b(ii):
Calculate the ratio .
-
20N.1.HL.TZ0.24:
The mass of nuclear fuel in a nuclear reactor decreases at the rate of every hour. The overall reaction process has an efficiency of . What is the maximum power output of the reactor?
A.
B.
C.
D.
- 20N.2.SL.TZ0.6a(i): State what is meant by binding energy of a nucleus.
- 20N.2.SL.TZ0.6a(ii): Outline why quantities such as atomic mass and nuclear binding energy are often expressed in...
-
20N.2.SL.TZ0.6a(iii):
Show that the energy released in the reaction is about .
- 20N.2.HL.TZ0.6a(i): State what is meant by binding energy of a nucleus.
- 20N.2.HL.TZ0.6a(ii): Outline why quantities such as atomic mass and nuclear binding energy are often expressed in...
-
20N.2.HL.TZ0.6a(iii):
Show that the energy released in the reaction is about .
- 21M.2.HL.TZ1.7d.i: Outline why high temperatures are required for fusion to occur.
- 21M.2.HL.TZ1.7d.ii: Outline, with reference to the graph, why energy is released both in fusion and in fission.
-
21M.2.HL.TZ1.7d.iii:
Uranium-235 is used as a nuclear fuel. The fission of uranium-235 can produce krypton-89 and barium-144.
Determine, in MeV and using the graph, the energy released by this fission.
- 21M.1.SL.TZ1.25: What is the relation between the value of the unified atomic mass unit in grams and the value of...
- 21M.1.HL.TZ1.22: In a hydrogen atom, the sum of the masses of a proton and of an electron is larger than the mass...
-
21M.1.HL.TZ2.22:
During the nuclear fission of nucleus X into nucleus Y and nucleus Z, energy is released. The binding energies per nucleon of X, Y and Z are , and respectively. What is true about the binding energy per nucleon of X, Y and Z?
A. > and >B. = and =
C. > and >
D. = +
- 21M.2.SL.TZ1.5c.i: Outline why high temperatures are required for fusion to occur
- 21M.2.SL.TZ1.5c.ii: Outline, with reference to the graph, why energy is released both in fusion and in fission.
-
21M.2.SL.TZ1.5c.iii:
Uranium-235 () is used as a nuclear fuel. The fission of uranium-235 can produce krypton-89 and barium-144.
Determine, in MeV and using the graph, the energy released by this fission.
-
21N.1.SL.TZ0.25:
The mass of a nucleus of iron-56 () is M.
What is the mass defect of the nucleus of iron-56?
A. M − 26mp − 56mn
B. 26mp + 30mn − M
C. M − 26mp − 56mn − 26me
D. 26mp + 30mn + 26me − M
- 21N.2.SL.TZ0.5a.i: State what is meant by the binding energy of a nucleus.
-
21N.2.SL.TZ0.5a.ii:
Draw, on the axes, a graph to show the variation with nucleon number of the binding energy per nucleon, . Numbers are not required on the vertical axis.
-
21N.2.SL.TZ0.5a.iii:
Identify, with a cross, on the graph in (a)(ii), the region of greatest stability.
-
21N.2.SL.TZ0.5b.i:
Show that the energy released in this decay is about 6 MeV.
-
21N.2.SL.TZ0.5b.ii:
The plutonium nucleus is at rest when it decays.
Calculate the ratio .
- 21N.2.HL.TZ0.4a.i: State what is meant by the binding energy of a nucleus.
-
21N.2.HL.TZ0.4a.ii:
Draw, on the axes, a graph to show the variation with nucleon number of the binding energy per nucleon, . Numbers are not required on the vertical axis.
-
21N.2.HL.TZ0.4a.iii:
Identify, with a cross, on the graph in (a)(ii), the region of greatest stability.
-
21N.2.HL.TZ0.4b.i:
Show that the energy released in this decay is about 6 MeV.
-
21N.2.HL.TZ0.4b.ii:
The plutonium nucleus is at rest when it decays.
Calculate the ratio .
-
22M.1.HL.TZ2.24:
A neutron is absorbed by a nucleus of uranium-235. One possible outcome is the production of two nuclides, barium-144 and krypton-89.
How many neutrons are released in this reaction?
A. 0
B. 1
C. 2
D. 3
7.3 – The structure of matter
- 16N.1.SL.TZ0.27: As quarks separate from each other within a hadron, the interaction between them becomes larger....
-
16N.2.SL.TZ0.4a:
A particular K meson has a quark structure s. State the charge on this meson.
-
16N.2.HL.TZ0.4a:
A particular K meson has a quark structure s. State the charge, strangeness and baryon number for this meson.
-
16N.2.HL.TZ0.4b:
The Feynman diagram shows the changes that occur during beta minus (β–) decay.
Label the diagram by inserting the four missing particle symbols and the direction of the arrows for the decay particles.
- 17M.1.SL.TZ1.15: Two pulses are travelling towards each other. What is a possible pulse shape when the pulses...
- 17M.2.SL.TZ1.5a: State the quark structures of a meson and a baryon.
-
17M.2.SL.TZ1.5b.ii:
Draw arrow heads on the lines representing and d in the .
- 17M.2.SL.TZ1.5b.iii: Identify the exchange particle in this decay.
- 17M.2.SL.TZ1.5c: Outline one benefit of international cooperation in the construction or use of high-energy...
-
17M.1.SL.TZ2.27:
The reaction p+ + n0 → p+ + 0 does not occur because it violates the conservation law of
A. electric charge.
B. baryon number.
C. lepton number.
D. strangeness.
- 17N.1.SL.TZ0.25: The Feynman diagram shows a particle interaction involving a W– boson. Which particles are...
- 17N.2.SL.TZ0.2b: Distinguish between hadrons and leptons.
-
18M.1.SL.TZ1.24:
Which Feynman diagram shows beta-plus (β+) decay?

-
18M.2.SL.TZ2.6a:
Rutherford constructed a model of the atom based on the results of the alpha particle scattering experiment. Describe this model.
-
18M.2.SL.TZ2.6c.i:
Draw a labelled arrow to complete the Feynman diagram.
-
18M.2.SL.TZ2.6c.ii:
Identify particle V.
- 18M.1.HL.TZ1.21: What is correct about the Higgs Boson? A. It was predicted before it was observed. B. ...
- 18M.1.HL.TZ2.20: Identify the conservation law violated in the proposed reaction. ...
-
18N.1.SL.TZ0.26:
Copper () decays to nickel (). What are the particles emitted and the particle that mediates the interaction?
-
18N.1.SL.TZ0.27:
The following interaction is proposed between a proton and a pion.
p+ + – → K– + +
The quark content of the – is ūd and the quark content of the K– is ūs.
Three conservation rules are considered
I. baryon number
II. charge
III. strangeness.
Which conservation rules are violated in this interaction?
A. I and II only
B. I and III only
C. II and III only
D. I, II and III
- 18N.1.HL.TZ0.20: In the Rutherford-Geiger-Marsden scattering experiment it was observed that a small percentage of...
-
18N.1.HL.TZ0.22:
The following decay is observed.
μ− → e− + vμ + X
What is particle X?
A. γ
B. e
C. Z0
D. ve
- 18N.1.HL.TZ0.38: Which is the correct Feynman diagram for pair annihilation and pair production?
-
19M.2.SL.TZ1.2a.ii:
Sketch the Feynman diagram that represents this reaction. The diagram has been started for you.
-
19M.2.SL.TZ1.2a.iii:
Energy is transferred to a hadron in an attempt to separate its quarks. Describe the implications of quark confinement for this situation.
- 19M.2.SL.TZ1.2b: The Standard Model was accepted by many scientists before the observation of the Higgs boson was...
-
19M.1.HL.TZ2.34:
The meson contains an up () quark. What is the quark structure of the meson?
A.
B.
C.
D.
-
19M.1.SL.TZ2.26:
Three conservation laws in nuclear reactions are
I. conservation of charge
II. conservation of baryon number
III. conservation of lepton number.
The reaction
is proposed.
Which conservation laws are violated in the proposed reaction?
A. I and II only
B. I and III only
C. II and III only
D. I, II and III
- 19M.1.SL.TZ2.27: Which Feynman diagram shows the emission of a photon by a charged antiparticle?
- 19N.1.SL.TZ0.27: What is correct about the nature and range of the strong interaction between nuclear...
-
20N.1.SL.TZ0.30:
The Feynman diagram shows some of the changes in a proton–proton collision.
What is the equation for this collision?
A.
B.
C.
D.
-
21M.2.HL.TZ1.7b:
Thallium-206 decays into lead-206 .
Identify the quark changes for this decay.
-
21M.2.SL.TZ1.7b:
When a pi meson π- (du̅) and a proton (uud) collide, a possible outcome is a sigma baryon Σ0 (uds) and a kaon meson Κ0 (ds̅).
Apply three conservation laws to show that this interaction is possible. -
21M.2.HL.TZ2.4b:
The neutron number N and the proton number Z are not equal for the nuclide . Explain, with reference to the forces acting within the nucleus, the reason for this.
- 21M.1.HL.TZ1.22: In a hydrogen atom, the sum of the masses of a proton and of an electron is larger than the mass...
- 21M.1.HL.TZ1.23: Which Feynman diagram describes the annihilation of an electron and its antiparticle?
-
21M.1.SL.TZ1.27:
A particle reaction is
.
Which conservation law is violated by the reaction?
A. Baryon number
B. Charge
C. Lepton number
D. Momentum
- 21M.1.SL.TZ2.27: A kaon is made up of two quarks. What is the particle classification of a kaon? A. Exchange...
- 21M.1.SL.TZ2.28: Consider the Feynman diagram below. What is the exchange particle X? A. Lepton B. Gluon C....
-
21M.2.SL.TZ1.5b:
Thallium-206 decays into lead-206 .
Identify the quark changes for this decay.
-
21M.2.SL.TZ2.4b:
The neutron number N and the proton number Z are not equal for the nuclide . Explain, with reference to the forces acting within the nucleus, the reason for this.
- 21N.1.SL.TZ0.27: The Higgs boson was discovered in the Large Hadron Collider at CERN. Which statements are correct...
- 21N.1.SL.TZ0.26: A proton collides with an electron. What are the possible products of the collision? A. Two...
- 21N.1.HL.TZ0.22: The Feynman diagram shows an interaction between a proton and an electron. What is the charge...
- 22M.1.SL.TZ2.25: Three statements about electrons are: I. Electrons interact through virtual photons.II. ...
-
22M.2.SL.TZ1.5a:
Describe the quark structure of a baryon.
- 22M.2.SL.TZ1.5b: The Feynman diagram shows a possible decay of the K+ meson. Identify the interactions that are...
- 22M.2.SL.TZ1.5c: The K+ meson can decay as K+ → μ+ + vμ. State and explain the interaction that is responsible...
