| Date | November 2020 | Marks available | 1 | Reference code | 20N.2.sl.TZ0.4 |
| Level | SL | Paper | 2 | Time zone | TZ0 |
| Command term | Outline | Question number | 4 | Adapted from | N/A |
Question
Nickel catalyses the conversion of propanone to propan-2-ol.
Outline how a catalyst increases the rate of reaction.
Explain why an increase in temperature increases the rate of reaction.
Discuss, referring to intermolecular forces present, the relative volatility of propanone and propan-2-ol.
The diagram shows an unlabelled voltaic cell for the reaction
Label the diagram with the species in the equation.
Suggest a metal that could replace nickel in a new half-cell and reverse the electron flow. Use section 25 of the data booklet.
Describe the bonding in metals.
Nickel alloys are used in aircraft gas turbines. Suggest a physical property altered by the addition of another metal to nickel.
Markscheme
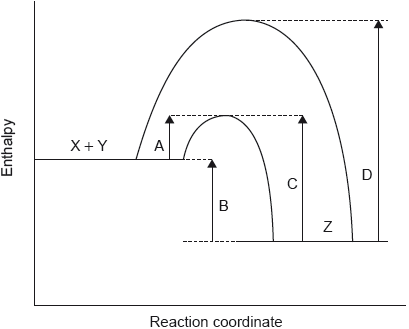
provides an alternative pathway/mechanism AND lower ✔
Accept description of how catalyst lowers (e.g. “reactants adsorb on surface «of catalyst»”, “reactant bonds weaken «when adsorbed»”).
more/greater proportion of molecules with ✔
greater frequency/probability/chance of collisions «between the molecules»
OR
more collision per unit of time/second ✔
hydrogen bonding/bonds «and dipole–dipole and London/dispersion forces are present in» propan-2-ol ✔
dipole–dipole «and London/dispersion are present in» propanone ✔
propan-2-ol less volatile AND hydrogen bonding/bonds stronger «than dipole–dipole »
OR
propan-2-ol less volatile AND «sum of all» intermolecular forces stronger ✔
✔
Accept OR .
electrostatic attraction ✔
between «a lattice of» metal/positive ions/cations AND «a sea of» delocalized electrons ✔
Accept “mobile/free electrons”.
Any of:
malleability/hardness
OR
«tensile» strength/ductility
OR
density
OR
thermal/electrical conductivity
OR
melting point
OR
thermal expansion ✔
Do not accept corrosion/reactivity or any chemical property.
Accept other specific physical properties.
Examiners report
A straight-forward question, however, half of the candidates only mentioned the lower activation energy and did not mention that this is through an alternative mechanism, so did not score the mark.
Half of the candidates gained the mark about the increased frequency of collision. Fewer candidates also clarified that a larger proportion of molecules have the activation energy.
Most candidates had the correct structure in their answers identifying the type of intermolecular forces in each compound and then comparing the strength of the two and reaching a conclusion. Some candidates did not know what was meant by volatile. Some candidates stated London dispersion forces in propanone instead of dipole-dipole.
60% of the candidates obtained the mark. Some candidates labelled the electrodes as ions indicating they do not understand the structure of a voltaic cell.
70% of the candidates answered correctly. The common mistake was to select a more reactive metal instead.
The mean mark on the question was 1.0 out of 2 marks. Mistakes included not mentioning the 'electrostatic attraction' and talking about 'nuclei attracting the delocalised electrons'. The weakest candidates discussed aspects of ionic and/or covalent bonding.
80% obtained the mark. Many candidates wrote more than one property, which should be discouraged. Incorrect answers included chemical properties such as reactivity.