| Date | November 2019 | Marks available | 2 | Reference code | 19N.3.sl.TZ0.1 |
| Level | SL | Paper | 3 | Time zone | TZ0 |
| Command term | Explain | Question number | 1 | Adapted from | N/A |
Question
A student investigated how the type of acid in acid deposition affects limestone, a building material mainly composed of calcium carbonate.
The student monitored the mass of six similarly sized pieces of limestone. Three were placed in beakers containing 200.0 cm3 of 0.100 mol dm−3 nitric acid, HNO3 (aq), and the other three in 200.0 cm3 of 0.100 mol dm−3 sulfuric acid, H2SO4 (aq).
The limestone was removed from the acid, washed, dried with a paper towel and weighed every day at the same time and then replaced in the beakers.
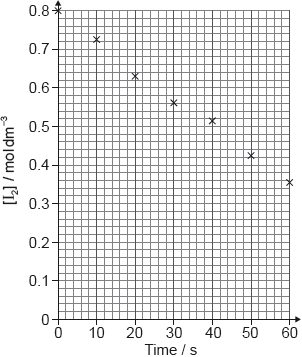
The student plotted the mass of one of the pieces of limestone placed in nitric acid against time.
[Source: © International Baccalaureate Organization 2019]
The student hypothesized that sulfuric acid would cause a larger mass loss than nitric acid.
Draw a best-fit line on the graph.
Determine the initial rate of reaction of limestone with nitric acid from the graph.
Show your working on the graph and include the units of the initial rate.
Explain why the rate of reaction of limestone with nitric acid decreases and reaches zero over the period of five days.
Suggest a source of error in the procedure, assuming no human errors occurred and the balance was accurate.
Justify this hypothesis.
The student obtained the following total mass losses.
She concluded that nitric acid caused more mass loss than sulfuric acid, which did not support her hypothesis.
Suggest an explanation for the data, assuming that no errors were made by the student.
Markscheme
best-fit smooth curve ✔
NOTE: Do not accept a series of connected lines that pass through all points OR any straight line representation.
tangent drawn at time zero ✔
g day−1 ✔
0.16 ✔
NOTE: Accept other reasonable units for initial rate eg, mol dm−3 s−1, mol dm−3 min−1, g s−1 OR g min−1.
M3 can only be awarded if the value corresponds to the correct unit given in M2.
Accept values for the initial rate for M3 in the range: 0.13 − 0.20 g day−1 OR 1.5 × 10−6 g s−1 − 2.3 × 10−6 g s−1 OR 7.5 × 10−8 − 1.2 × 10−7 mol dm−3 s−1 OR 4.5 × 10−6 − 6.9 × 10−6 mol dm−3 min−1 OR 9.0 × 10−5 − 1.4 × 10−4 g min−1 OR a range based on any other reasonable unit for rate.
Ignore any negative rate value.
Award [2 max] for answers such as 0.12/0.11 g day−1, incorrectly obtained by using the first two points on the graph (the average rate between t = 0 and 1 day).
Award [1 max] for correctly calculating any other average rate.
acid used up
OR
acid is the limiting reactant ✔
concentration of acid decreases
OR
less frequent collisions ✔
NOTE: Award [1 max] for "surface area decreases" if the idea that CaCO3 is used up/acts as the limiting reactant” is conveyed for M1.
Do not accept “reaction reaches equilibrium” for M2.
surface area not uniform
NOTE: Accept “acids impure.
OR
limestone pieces do not have same composition/source
NOTE: Accept “«limestone» contains impurities”.
OR
limestone absorbed water «which increased mass»
OR
acid removed from solution when limestone removed
NOTE: Accept “loss of limestone when dried" OR "loss of limestone due to crumbling when removed from beaker”.
OR
«some» calcium sulfate deposited on limestone lost
OR
pieces of paper towel may have stuck to limestone
OR
beakers not covered/evaporation
OR
temperature was not controlled ✔
sulfuric acid is diprotic/contains two H+ «while nitric acid contains one H+»/releases more H+ «so reacts with more limestone»
OR
higher concentration of protons/H+ ✔
NOTE: Ignore any reference to the relative strengths of sulfuric acid and nitric acid.
Accept “sulfuric acid has two hydrogens «whereas nitric has one»”.
Accept "dibasic" for "diprotic".
calcium sulfate remained/deposited on limestone «in sulfuric acid»
OR
reaction prevented/stopped by slightly soluble/deposited/layer of calcium sulfate ✔
NOTE: Answer must refer to calcium sulfate.