| Date | May 2019 | Marks available | 1 | Reference code | 19M.3.sl.TZ2.1 |
| Level | SL | Paper | 3 | Time zone | TZ2 |
| Command term | Identify | Question number | 1 | Adapted from | N/A |
Question
This question is about a mug made of a lead alloy.
The rate of lead dissolving in common beverages with various pH values was analysed.
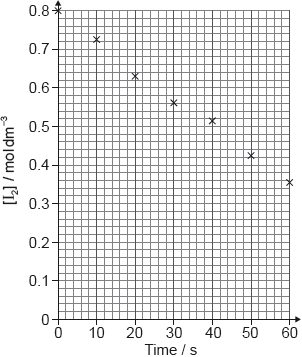
Identify the experiment with the highest rate of lead dissolving.
Suggest why the relationship between time and lead concentration for Cola at 16 °C is not linear.
Lead(II) chloride, PbCl2, has very low solubility in water.
PbCl2 (s) Pb2+ (aq) + 2Cl− (aq)
Explain why the presence of chloride ions in beverages affects lead concentrations.
A mean daily lead intake of greater than 5.0 × 10−6 g per kg of body weight results in increased lead levels in the body.
Calculate the volume, in dm3, of tap water from experiment 8 which would exceed this daily lead intake for an 80.0 kg man.
Markscheme
6 [✔]
Note: Accept “orange juice”.
equilibrium is being established «between lead in solution and in mug»
OR
solution becoming saturated
OR
concentration of lead ions/[Pb2+] has increased «over time»
OR
acid concentration has decreased «as reacted with lead»
OR
surface lead has decrease/formed a compound/forms insoluble layer on surface
OR
acid reacts with other metals «because it is an alloy» [✔]
Note: Do not accept “concentration of cola, orange juice, etc… has decreased”.
Do not accept responses that only discusses mathematical or proportional relationships.
equilibrium shifts to the left/towards reactants [✔]
lead «compounds/ions» precipitate
OR
concentration of lead «ions»/[Pb2+] decreases [✔]
Note: Award [2] for “equilibrium shifts to the left/towards reactants due to common ion effect”.
Accept “lead ions/[Pb2+] removed from solution” for M2.
«daily limit = 5.0 × 10–6 g kg–1 × 80.0kg =» 4.0 × 10–4 «g of lead» [✔]
«volume =» 2.7 × 10–2/0.027 «dm3» [✔]
Note: Award [2] for correct final answer.
Examiners report
Most candidates did well on this question, identifying the correct experiment by number or beverage.
Many candidates struggled with this question, answering it from a mathematical perspective rather than explaining why the rate would decrease over time from a chemical perspective. There were several possible correct answers (reaching equilibrium, acid concentration decreasing, solution becoming saturated with lead ions, etc.…)
This question was an equilibrium question. Many students received 1 mark for either concentration of lead decreased, or lead chloride was produced and quite a few recognized that the explanation was the reaction shifted to the reactant or left side for the second mark.
Most students receive one mark for this question, and many receive both marks. The most common mistakes involved incorrect conversions from gram to milligrams or milligrams to grams.