| Date | May 2013 | Marks available | 2 | Reference code | 13M.3.hl.TZ2.B3 |
| Level | HL | Paper | 3 | Time zone | TZ2 |
| Command term | Explain | Question number | B3 | Adapted from | N/A |
Question
The enzyme hexokinase catalyses one of the initial reactions between glucose and adenosine triphosphate (ATP) during the process of glycolysis.
The graph below shows how the rate of this enzyme-catalysed reaction changes as the glucose concentration is increased.
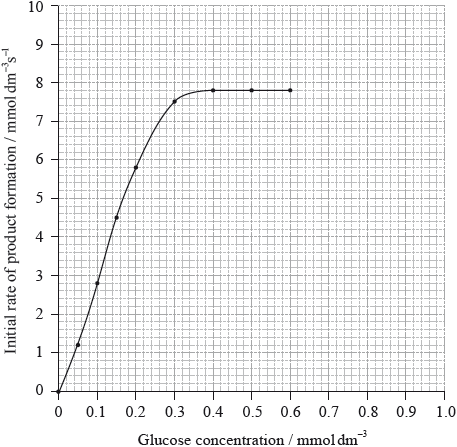
From the graph, determine \({V_{{\text{max}}}}\) and the Michaelis constant, \({K_{\text{m}}}\).
\({V_{{\text{max}}}}\):
\({K_{\text{m}}}\):
Explain why a low value of \({K_{\text{m}}}\) is significant.
State and explain the effect of a competitive inhibitor on the value of \){K_{\text{m}}}\).
Markscheme
\({\text{(}}{V_{{\text{max}}}}{\text{:) 7.75}}\)–\({\text{7.85 (mmol}}\,{\text{d}}{{\text{m}}^{ - 3}}{{\text{s}}^{ - 1}}{\text{)}}\);
\({\text{(}}{K_{\text{m}}}{\text{:) 0.12}}\)–\({\text{0.14 (mmol}}\,{\text{d}}{{\text{m}}^{ - 3}}{\text{)}}\);
Accept 7.8 for Vmax.
hexokinase/enzyme (with a low \({K_{\text{m}}}\)) has a high affinity for glucose/substrate / hexokinase/enzyme is saturated with substrate / hexokinase/ enzyme reacts quickly to form an ES complex which breaks down rapidly;
Do not accept strong binding/bonding between enzyme and substrate.
hence needs a lower concentration of glucose/substrate to achieve \({V_{{\text{max}}}}\) / a fast reaction occurs even when substrate concentration is low;
Accept “enzyme is efficient even at low substrate concentration”.
First mark:
\({K_{\text{m}}}\) increases;
Any two for final two marks:
inhibitor has similar structure to that of substrate;
hence inhibitor occupies/fits same active sites;
inhibitor does not affect \({V_{{\text{max}}}}\);
substrate must wait until the inhibitor leaves the active site;
reaction slows down/rate decreases;
Examiners report
The determination of Vmax and Km from the graph was done very well overall.
Answers were often poorly presented but many candidates scored the second mark. The high affinity of the enzyme for the substrate was rarely mentioned for the first marking point.
Many candidates obtained the first mark for \({K_{\text{m}}}\) increasing and a good number of candidates managed to achieve the last two marking points.

