| Date | May 2018 | Marks available | 2 | Reference code | 18M.3.hl.TZ1.4 |
| Level | HL | Paper | 3 | Time zone | TZ1 |
| Command term | Draw | Question number | 4 | Adapted from | N/A |
Question
Both HDPE (high density polyethene) and LDPE (low density polyethene) are produced by the polymerization of ethene.
An alternative method of polymerizing molecules is condensation polymerization. One of the earliest condensation polymers was nylon-6. A short section of the polymer chain of nylon-6 is shown below.
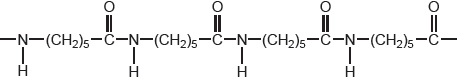
Draw the structure of the monomer from which nylon-6 is produced by a condensation reaction.
Deduce, giving a reason, whether the atom economy of a condensation polymerization, such as this, would be greater or less than an addition polymerization, such as the formation of HDPE.
Markscheme
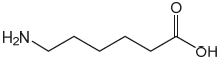
–NH2 AND –COOH
six C-atoms
Accept –COCl instead of –COOH.
[2 marks]
less AND a second molecule/product formed
Accept “not all the reactant molecules «in the equation» are converted «to product molecules»”.
[1 mark]

