| Date | May 2010 | Marks available | 2 | Reference code | 10M.3.hl.TZ1.C2 |
| Level | HL | Paper | 3 | Time zone | TZ1 |
| Command term | Write | Question number | C2 | Adapted from | N/A |
Question
Kevlar can be made by reacting 1,4-diaminobenzene, \({{\text{H}}_{\text{2}}}{\text{N}}{{\text{C}}_{\text{6}}}{{\text{H}}_{\text{4}}}{\text{N}}{{\text{H}}_{\text{2}}}\), with 1,4-benzenedicarbonyl chloride, \({\text{ClOC}}{{\text{C}}_{\text{6}}}{{\text{H}}_{\text{4}}}{\text{COCl}}\). Write the equation for the reaction of n molecules of 1,4-diaminobenzene reacting with n molecules of 1,4-benzenedicarbonyl chloride.
Kevlar is an example of a lyotropic liquid crystal. Outline what is meant by lyotropic liquid crystal.
Markscheme
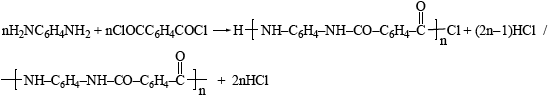
correct products;
correctly balanced;
liquid crystals:
fluids with properties that depend on the molecular orientation relative to a fixed axis;
lyotropic:
solutions that show the liquid-crystal state at certain concentrations;
Examiners report
Few candidates could give the correct equation to produce Kevlar, though some scored 1 mark for giving the correct products.
The concept of liquid crystals was not understood by many. Very few mentioned that lyotropic is a solution.

