| Date | November 2011 | Marks available | 2 | Reference code | 11N.3.hl.TZ0.C2 |
| Level | HL | Paper | 3 | Time zone | TZ0 |
| Command term | Explain | Question number | C2 | Adapted from | N/A |
Question
Since the accidental discovery of polyethene in the 1930s, polymers have played an essential role in daily life because of their wide range of properties and uses.
Polyurethanes are made from dialcohol (diol) and diisocyanate monomers. By considering the structures of the two monomers and the repeating unit of the polymer given below, suggest why it could be argued that this reaction is not an example of a condensation polymer.
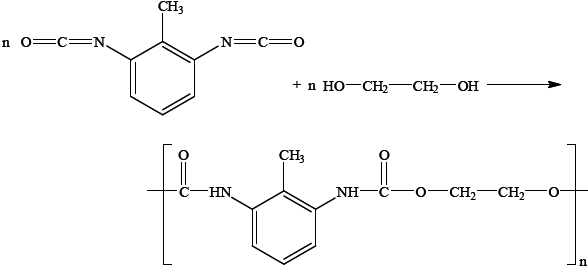
Kevlar is another example of a condensation polymer. Explain how the great strength of Kevlar depends on its structure.
Markscheme
all atoms in the two monomers end up in the polymer / it is an addition reaction;
condensation should involve loss of a (small) molecule / no loss of small molecule in the reaction / OWTTE;
Accept water in place of (small) molecule.
chains have trans orientation / close approach between straight chains / chains have polar (N–H and C=O) groups / amide linkages (which are aligned) / OWTTE;
H-bonding between chains (give it great strength);
Examiners report
Most did not correctly answer why the polymer given was not an example of condensation polymer, namely condensation should involve loss of a small molecule whereas all atoms in the two monomers end up in the polymer (and thus it is an addition polymer).
Hydrogen bonding between chains in Kevlar was well known, but almost none scored the mark for recognizing chains have cis orientation, making close approach possible.

