| Date | November 2012 | Marks available | 1 | Reference code | 12N.3.hl.TZ0.C4 |
| Level | HL | Paper | 3 | Time zone | TZ0 |
| Command term | State | Question number | C4 | Adapted from | N/A |
Question
\[{\text{n }}{{\text{H}}_2}{\text{N}}{{\text{C}}_6}{{\text{H}}_4}{\text{N}}{{\text{H}}_2} + {\text{n ClOC}}{{\text{C}}_6}{{\text{H}}_4}{\text{COCl}} \to {\rm{H\rlap{-} [HN}}{{\text{C}}_6}{{\text{H}}_4}{\text{NHOC}}{{\text{C}}_6}{{\text{H}}_4}{\text{CO}}{{\rm{\rlap{-} ]}}_{\text{n}}}{\text{Cl}} + {\text{2(n}} - {\text{1)HCl}}\]
The following diagram shows two adjacent molecules in a sample of solid Kevlar.
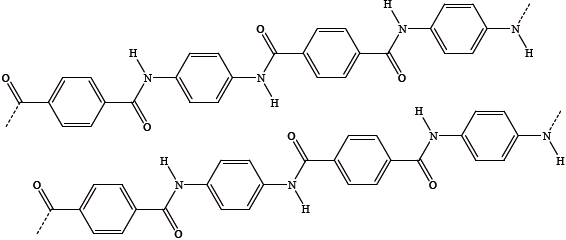
Kevlar is very unreactive but dissolves in concentrated sulfuric acid.
State the type of polymerization involved.
(i) Identify the strongest type of intermolecular force between the two molecules.
(ii) Annotate the diagram (above) by adding dotted lines to show the strongest intermolecular forces.
Kevlar is five times as strong as steel, partly due to its strong intermolecular forces. State another feature of the molecules which gives Kevlar such great strength.
(i) Suggest how the sulfuric acid is able to separate the Kevlar chains.
(ii) Evaluate the long-term environmental impact of Kevlar.
Markscheme
condensation (polymerization);
(i) hydrogen bonding;
(ii) 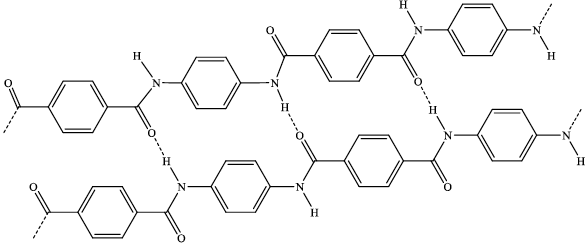
dotted lines showing intermolecular forces;
molecules are straight and inflexible/rod-like;
(i) sulfuric acid protonates N and O atoms in the amide groups / hydrolyses/breaks down amide groups;
which weakens/breaks hydrogen bonds between the chains (allowing it to dissolve);
(ii) non-biodegradable;
so would take up space in landfill;
inefficient combustion could lead to the formation of dioxins/pollutants/toxic compounds;
Examiners report
Option C was one of the least popular options. A surprising number did not know the purpose of the raw materials put into the blast furnace, some thinking scrap iron was a common raw material. There were few correct equations, but many would have been mark-worthy had they been balanced. Very few candidates appreciated the role of limestone or calcium oxide in neutralising the acidic impurities in iron ore, i.e. silica, and could write the equation for the formation of calcium silicate slag. Most candidates knew that reducing the carbon content of iron to form steel makes it less brittle, but a substantially lower proportion gave annealing, while others threw in tempering and quenching for good measure. Annealing continues to be a challenging notion for many students. Sections C2 and C4 of the syllabus account for 3.5 hours of study but candidates often seemed to be guessing whether the processes in question C2 used homogeneous or heterogeneous catalysts. The properties of polymers were generally well known but some of the explanations were a bit thin. The solubility of Kevlar in sulphuric acid was little known but a substantial number of candidates were able to state that it is non-biodegradable, and/or that an inefficient combustion leads to the formation of pollutants. Only stronger candidates identified dioxins as pollutant. The connection with landfills was rarely seen.
Option C was one of the least popular options. A surprising number did not know the purpose of the raw materials put into the blast furnace, some thinking scrap iron was a common raw material. There were few correct equations, but many would have been mark-worthy had they been balanced. Very few candidates appreciated the role of limestone or calcium oxide in neutralising the acidic impurities in iron ore, i.e. silica, and could write the equation for the formation of calcium silicate slag. Most candidates knew that reducing the carbon content of iron to form steel makes it less brittle, but a substantially lower proportion gave annealing, while others threw in tempering and quenching for good measure. Annealing continues to be a challenging notion for many students. Sections C2 and C4 of the syllabus account for 3.5 hours of study but candidates often seemed to be guessing whether the processes in question C2 used homogeneous or heterogeneous catalysts. The properties of polymers were generally well known but some of the explanations were a bit thin. The solubility of Kevlar in sulphuric acid was little known but a substantial number of candidates were able to state that it is non-biodegradable, and/or that an inefficient combustion leads to the formation of pollutants. Only stronger candidates identified dioxins as pollutant. The connection with landfills was rarely seen.
Option C was one of the least popular options. A surprising number did not know the purpose of the raw materials put into the blast furnace, some thinking scrap iron was a common raw material. There were few correct equations, but many would have been mark-worthy had they been balanced. Very few candidates appreciated the role of limestone or calcium oxide in neutralising the acidic impurities in iron ore, i.e. silica, and could write the equation for the formation of calcium silicate slag. Most candidates knew that reducing the carbon content of iron to form steel makes it less brittle, but a substantially lower proportion gave annealing, while others threw in tempering and quenching for good measure. Annealing continues to be a challenging notion for many students. Sections C2 and C4 of the syllabus account for 3.5 hours of study but candidates often seemed to be guessing whether the processes in question C2 used homogeneous or heterogeneous catalysts. The properties of polymers were generally well known but some of the explanations were a bit thin. The solubility of Kevlar in sulphuric acid was little known but a substantial number of candidates were able to state that it is non-biodegradable, and/or that an inefficient combustion leads to the formation of pollutants. Only stronger candidates identified dioxins as pollutant. The connection with landfills was rarely seen.
Option C was one of the least popular options. A surprising number did not know the purpose of the raw materials put into the blast furnace, some thinking scrap iron was a common raw material. There were few correct equations, but many would have been mark-worthy had they been balanced. Very few candidates appreciated the role of limestone or calcium oxide in neutralising the acidic impurities in iron ore, i.e. silica, and could write the equation for the formation of calcium silicate slag. Most candidates knew that reducing the carbon content of iron to form steel makes it less brittle, but a substantially lower proportion gave annealing, while others threw in tempering and quenching for good measure. Annealing continues to be a challenging notion for many students. Sections C2 and C4 of the syllabus account for 3.5 hours of study but candidates often seemed to be guessing whether the processes in question C2 used homogeneous or heterogeneous catalysts. The properties of polymers were generally well known but some of the explanations were a bit thin. The solubility of Kevlar in sulphuric acid was little known but a substantial number of candidates were able to state that it is non-biodegradable, and/or that an inefficient combustion leads to the formation of pollutants. Only stronger candidates identified dioxins as pollutant. The connection with landfills was rarely seen.

