| Date | May 2013 | Marks available | 2 | Reference code | 13M.3.hl.TZ2.C3 |
| Level | HL | Paper | 3 | Time zone | TZ2 |
| Command term | Draw | Question number | C3 | Adapted from | N/A |
Question
Another polymer that has cross-linking is Kevlar. Kevlar can be made by reacting 1,4-diaminobenzene with benzene-1,4-dicarboxylic acid.
Draw the structural formula of the repeating unit in Kevlar.
Explain how the long rigid chains in Kevlar are able to form cross-links to build up a three-dimensional structure.
Markscheme
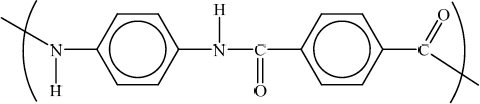
Award [1] for correct showing of amide linkage.
Award [1] for remainder of structure correct.
Accept H and O both up or both down or accept –NH–CO– but do not accept –N–H–CO–.
Brackets not necessary to gain marks.
the \({\delta ^ - }\)/lone pair on the O of the C=O and the \({\delta ^ + }\) on the H of the N–H / the polarity of the C=O and the N–H bonds;
Accept N-H of one chain and C=O of adjacent chain or NH2 group of one chain with C=O group of another chain.
(form) hydrogen bonds / OWTTE;
Accept suitable diagram.
Examiners report
There were very few correct answers for this question with many blank answer boxes. In a) i), only a small number of candidates gave correct answers here. Many incorrectly gave the group –CHO instead of –CH2OH as a substituent on the ring.
There were very few correct answers for this question with many blank answer boxes. In a) i), only a small number of candidates gave correct answers here. Many incorrectly gave the group –CHO instead of –CH2OH as a substituent on the ring.

