 To understand electrical currents, we must first understand what an electric current consists of and what makes it 'go'.
To understand electrical currents, we must first understand what an electric current consists of and what makes it 'go'.
An electric current is the movement of charges. Charges move when they experience an electric force. While charge is the more axiomatic quantity, current is the fundamental quantity with an SI base unit.
Key Concepts
Charge is a conserved quantity, like mass. It is measured in Coulombs. The smallest amount of charge that exists independently is 1.6 x 10 -19 C; this is called the fundamental unit of charge and is the charge associated with the components of the atom. All matter contains charge and all things that are charged have mass; they are inseperable.
However, unlike mass (which is always attractive across the universe), charges fall into one of two categories: positive and negative.
An atom is uncharged as it contains equal numbers of positive charges and negative charges:
- The nucleus is charged by positive protons, with a charge of +1.6 x 10-19 C.
- The outer shells are charged by negative electrons, with a charge of -1.6 x 10-19 C.
- The number of protons = number of electrons.
- Neutrons, also contained in the nucleus, are uncharged.
- A particle that has electrons added or removed is called an ion, a charged particle.
Coulombs law tells us which quantities affect the size of the electric force between two point charges (or between charges that can be modelled as having all of the charge at their centres).

The force experienced by two point charges is directly proportional to the product of their charges and inversely proportional to their separation squared:
\(F_E \propto {{q_1 q_2}\over r^2}\)
The proportional sign can be replaced with an equals sign by multiplying by a constant:
\(F_E=k{{q_1 q_2}\over r^2}\)

NB: This is similar to Newton's law of gravitation.
How much of Electric fields have you understood?


 As with graviatational forces (see
As with graviatational forces (see 
 How could you use field lines to show the repulsion of two charges?
How could you use field lines to show the repulsion of two charges?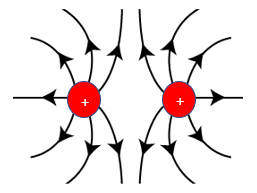
 The significance of the test charge is that the electric field strength is calculated as though the placed charge were not there (and indeed, the size of this charge cancels out!). However, if a charge were actually placed there, it would change the shape of the field in that location.
The significance of the test charge is that the electric field strength is calculated as though the placed charge were not there (and indeed, the size of this charge cancels out!). However, if a charge were actually placed there, it would change the shape of the field in that location. Twitter
Twitter  Facebook
Facebook  LinkedIn
LinkedIn