DP Chemistry Questionbank

Topic 5: Energetics/thermochemistry
Description
[N/A]Directly related questions
-
16N.1.sl.TZ0.1:
Which change of state is exothermic?
A. CO2(s) → CO2(g)
B. H2O(l) → H2O(g)
C. NH3(g) → NH3(l)
D. Fe(s) → Fe(l) -
20N.1.hl.TZ0.15:
Which statements about bond strength and activation energy are correct for this reaction?
-
20N.2.hl.TZ0.3b:
Calculate the standard enthalpy change, , for this reaction using section 12 of the data booklet.
-
17M.1.sl.TZ1.13:
Which expression gives the mass, in g, of ethanol required to produce 683.5 kJ of heat upon complete combustion?
(Mr for ethanol = 46.0, )
A.
B.
C.
D.
-
17M.2.sl.TZ1.4g:
The standard enthalpy of formation of N2H4(l) is +50.6 kJmol−1. Calculate the enthalpy of vaporization, ΔHvap, of hydrazine in kJmol−1.
N2H4(l) → N2H4(g)
(If you did not get an answer to (f), use −85 kJ but this is not the correct answer.)
-
17M.3.sl.TZ1.5c:
Suggest how heat loss could be reduced.
- 17M.1.sl.TZ2.13: What can be deduced from this reaction profile? A. The reactants are less stable than the...
- 17M.1.sl.TZ2.15: What can be deduced from the facts that ozone absorbs UV radiation in the region of 340 nm...
-
17N.2.hl.TZ0.3b:
Explain why the melting points of the group 1 metals (Li → Cs) decrease down the group whereas the melting points of the group 17 elements (F → I) increase down the group.
- 17N.1.hl.TZ0.19: The enthalpy change for the dissolution of NH4NO3 is +26 kJ mol–1 at 25 °C. Which statement...
-
17N.1.sl.TZ0.13:
Which statement is correct for this reaction?
Fe2O3 (s) + 3CO (g) → 2Fe (s) + 3CO2 (g) ΔH = −26.6 kJ
A. 13.3 kJ are released for every mole of Fe produced.
B. 26.6 kJ are absorbed for every mole of Fe produced.
C. 53.2 kJ are released for every mole of Fe produced.
D. 26.6 kJ are released for every mole of Fe produced.
-
21M.1.sl.TZ1.14:
What is the enthalpy change, in J, when 5 g of water is heated from 10°C to 18°C?
Specific heat capacity of water: 4.18 kJ kg−1 K−1
A. 5 × 4.18 × 8
B. 5 × 10−3 × 4.18 × 8
C. 5 × 4.18 × (273 + 8)
D. 5 × 10−3 × 4.18 × (273 + 8)
-
21M.2.sl.TZ1.3c:
Iron has a relatively small specific heat capacity; the temperature of a 50 g sample rises by 44.4°C when it absorbs 1 kJ of heat energy.
Determine the specific heat capacity of iron, in J g−1 K−1. Use section 1 of the data booklet.
-
21M.2.hl.TZ1.7c:
Explain, using equations, how the presence of results in a chain reaction that decreases the concentration of ozone in the stratosphere.
-
18N.1.sl.TZ0.15:
Consider the following reaction:
N2 (g) + 3H2 (g) 2NH3 (g)
Which calculation gives ΔHΘ, in kJ, for the forward reaction?
A. 2z − y − 3x
B. y + 3x − 2z
C. y + 3x − 6z
D. 6z − y − 3x
-
18N.2.sl.TZ0.1b.i:
The reaction was carried out in a calorimeter. The maximum temperature rise of the solution was 7.5 °C.
Calculate the enthalpy change, ΔH, of the reaction, in kJ, assuming that all the heat released was absorbed by the solution. Use sections 1 and 2 of the data booklet.
- 22M.1.sl.TZ2.13: What is correct about energy changes during bond breaking and bond formation?
-
22M.2.hl.TZ1.3b(ii):
Outline why the value obtained in (b)(i) might differ from a value calculated using ΔHf data.
-
22M.2.hl.TZ2.8f(i):
Calculate the enthalpy change of the reaction, ΔH, using section 11 of the data booklet.
-
19M.3.hl.TZ1.2b(iv):
Predict, giving a reason, how the final enthalpy of reaction calculated from this experiment would compare with the theoretical value.
- 19M.1.hl.TZ1.14: When equal masses of X and Y absorb the same amount of energy, their temperatures rise by 5 °C...
-
19M.2.sl.TZ2.1c(iii):
Determine the enthalpy change for the reaction, in kJ, to produce A using section 11 of the data booklet.
- 19N.3.hl.TZ0.18a(ii): Explain why fusion is an exothermic process.
-
16N.2.sl.TZ0.1a:
Ethane-1,2-diol can be formed according to the following reaction.
2CO (g) + 3H2 (g) HOCH2CH2OH (g)
(i) Deduce the equilibrium constant expression, Kc, for this reaction.
(ii) State how increasing the pressure of the reaction mixture at constant temperature will affect the position of equilibrium and the value of Kc.
Position of equilibrium:
Kc:
(iii) Calculate the enthalpy change, ΔHθ, in kJ, for this reaction using section 11 of the data booklet. The bond enthalpy of the carbon–oxygen bond in CO (g) is 1077kJmol-1.
(iv) The enthalpy change, ΔHθ, for the following similar reaction is –233.8 kJ.
2CO(g) + 3H2(g) HOCH2CH2OH (l)
Deduce why this value differs from your answer to (a)(iii).
-
16N.1.sl.TZ0.13:
Hydrazine reacts with oxygen.
N2H4(l) + O2(g) → N2(g) + 2H2O(l) ΔHθ = -623 kJ
What is the standard enthalpy of formation of N2H4(l) in kJ? The standard enthalpy of formation of H2O(l) is -286 kJ.
A. -623 - 286
B. -623 + 572
C. -572 + 623
D. -286 + 623 -
20N.1.sl.TZ0.14:
Which combination will give you the enthalpy change for the hydrogenation of ethene to ethane, ?
A.
B.
C.
D.
-
17M.3.sl.TZ1.5a:
Explain how the concentration may be calculated in this way.
-
17M.2.sl.TZ2.5b:
Nitrogen dioxide and carbon monoxide react according to the following equation:
NO2(g) + CO(g) NO(g) + CO2(g) ΔH = –226 kJ
Calculate the activation energy for the reverse reaction.
-
17N.1.hl.TZ0.17:
The combustion of glucose is exothermic and occurs according to the following equation:
C6H12O6 (s) + 6O2 (g) → 6CO2 (g) + 6H2O (g)
Which is correct for this reaction?
- 21M.1.sl.TZ1.28: The enthalpy of combustion of a fuel was determined using the calorimeter shown. The final result...
- 21M.1.hl.TZ2.15: The potential energy profile of a reaction is shown. What can be determined about stability...
-
21M.2.hl.TZ1.4d(i):
Determine the enthalpy change, ΔH, in kJ. Use section 11 of the data booklet.
Bond enthalpy of CO = 1077 kJ mol−1.
-
21M.2.sl.TZ2.1b:
Thermodynamic data for the decomposition of calcium carbonate is given.
Calculate the enthalpy change of reaction, ΔH, in kJ, for the decomposition of calcium carbonate.
-
18M.2.sl.TZ2.4b.i:
Outline why no value is listed for H2(g).
-
21N.1.sl.TZ0.15:
Which equation represents the standard enthalpy of formation of lithium oxide?
A. 4Li (s) + O2 (g) → 2Li2O (s)B. 2Li (s) + O2 (g) → Li2O (s)
C. Li (s) + O2 (g) → Li2O (s)
D. Li (g) + O2 (g) → Li2O (g)
-
18N.2.sl.TZ0.1b.ii:
State another assumption you made in (b)(i).
-
18N.2.sl.TZ0.7c:
Determine the standard enthalpy change, ΔHΘ, of step 1.
-
18N.2.sl.TZ0.7b:
Calculate the standard enthalpy change, ΔHΘ, of step 2 using section 13 of the data booklet.
-
22M.2.sl.TZ1.2d(iii):
Suggest why the values obtained in (d)(i) and (d)(ii) differ.
-
19M.2.sl.TZ2.3a:
Outline why ozone in the stratosphere is important.
-
19M.1.sl.TZ2.14:
Methane undergoes incomplete combustion.
2CH4 (g) + 3O2 (g) → 2CO (g) + 4H2O (g)
What is the enthalpy change, in kJ, using the bond enthalpy data given below?
A. [2(1077) + 4(463)] − [2(414) + 3(498)]
B. [2(414) + 3(498)] − [2(1077) + 4(463)]
C. [8(414) + 3(498)] − [2(1077) + 8(463)]
D. [2(1077) + 8(463)] − [8(414) + 3(498)]
-
19N.1.sl.TZ0.15:
What is the enthalpy change of the reaction?
C6H14 (l) → C2H4 (g) + C4H10 (g)
A. + 1411 + 2878 + 4163
B. + 1411 − 2878 − 4163
C. + 1411 + 2878 − 4163
D. − 1411 − 2878 + 4163
-
19N.1.sl.TZ0.13:
What is the enthalpy of combustion, ΔHc, of ethanol in kJ mol−1?
Maximum temperature of water: 30.0°C
Initial temperature of water: 20.0°C
Mass of water in beaker: 100.0 g
Loss in mass of ethanol: 0.230 g
Mr (ethanol): 46.08
Specific heat capacity of water: 4.18 J g−1 K−1
q = mcΔTA.
B.
C.
D.
- 19N.1.sl.TZ0.14: Which quantity is likely to be the most inaccurate due to the sources of error in this...
-
20N.2.sl.TZ0.3b:
Calculate the standard enthalpy change, , for this reaction using section 12 of the data booklet.
-
17M.3.sl.TZ1.5d:
State one other assumption that is usually made in the calculation of the heat produced.
-
17M.1.sl.TZ2.14:
Why is the value of the enthalpy change of this reaction calculated from bond enthalpy data less accurate than that calculated from standard enthalpies of formation?
2C2H6(g) + 7O2(g) → 4CO2(g) + 6H2O(g)
A. All the reactants and products are gases.
B. Bond enthalpy data are average values for many compounds.
C. Elements do not have standard enthalpy of formation.
D. Standard enthalpies of formation are per mole.
-
17N.2.sl.TZ0.1d.i:
Determine the heat change, q, in kJ, for the neutralization reaction between ethanoic acid and sodium hydroxide.
Assume the specific heat capacities of the solutions and their densities are those of water.
-
21M.2.sl.TZ1.4d(i):
Determine the enthalpy change, ΔH, in kJ. Use section 11 of the data booklet.
Bond enthalpy of CO = 1077 kJ mol−1.
- 21M.2.sl.TZ2.1c(i): The potential energy profile for a reaction is shown. Sketch a dotted line labelled “Catalysed”...
- 18M.1.sl.TZ1.13: The enthalpy of combustion of ethanol is determined by heating a known mass of tap water in a...
-
18M.1.sl.TZ2.14:
What is the enthalpy change of combustion of urea, (NH2)2CO, in kJ mol−1?
2(NH2)2CO(s) + 3O2(g) → 2CO2(g) + 2N2(g) + 4H2O(l)
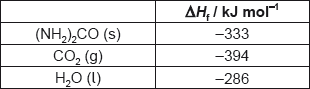
A. 2 × (−333) −2 × (−394) −4 × (−286)
B. [2 × (−394) + 4 × (−286) −2 × (−333)]
C. 2 × (−394) + 4 × (−286) −2 × (−333)
D. [2 × (−333) −2 × (−394) −4 × (−286)]
-
18M.2.sl.TZ2.4a:
Hydrogen gas can be formed industrially by the reaction of natural gas with steam.
CH4(g) + H2O(g) → 3H2(g) + CO(g)
Determine the enthalpy change, ΔH, for the reaction, in kJ, using section 11 of the data booklet.
Bond enthalpy for C≡O: 1077 kJ mol−1
- 21N.2.sl.TZ0.4a(iii): Suggest, with a reason, why 1-iodopentane reacts faster than 1-chloropentane under the same...
- 22M.1.sl.TZ1.14: What is the enthalpy change of the following reaction? CH2CHCH2CH3 + HBr → CH3CHBrCH2CH3 A. ...
-
18N.1.sl.TZ0.13:
Consider the following reactions:
Fe2O3 (s) + CO (g) → 2FeO (s) + CO2 (g) ΔHΘ = −3 kJ
Fe (s) + CO2 (g) → FeO (s) + CO (g) ΔHΘ = +11 kJ
What is the ΔHΘ value, in kJ, for the following reaction?
Fe2O3 (s) + 3CO (g) → 2Fe (s) + 3CO2 (g)
A. −25
B. −14
C. +8
D. +19
-
19M.3.hl.TZ1.2b(iii):
The formula q = mcΔT was used to calculate the energy released. The values used in the calculation were m = 25.00 g, c = 4.18 J g−1 K−1.
State an assumption made when using these values for m and c.
-
19M.3.hl.TZ1.2a(ii):
State what point Y on the graph represents.
-
19M.3.sl.TZ1.2b(i):
The maximum temperature used to calculate the enthalpy of reaction was chosen at a point on the extrapolated (dotted) line.
State the maximum temperature which should be used and outline one assumption made in choosing this temperature on the extrapolated line.
Maximum temperature:
Assumption:
- 19M.1.sl.TZ1.13: When equal masses of X and Y absorb the same amount of energy, their temperatures rise by 5 °C...
- 19M.1.sl.TZ1.14: What is the enthalpy change of reaction for the following equation? A. x + y + z B. −x − y +...
-
19M.1.sl.TZ2.13:
Consider the following equations.
2Al (s) + O2 (g) → Al2O3 (s) ΔHƟ = −1670 kJ
Mn (s) + O2 (g) → MnO2 (s) ΔHƟ = −520 kJWhat is the standard enthalpy change, in kJ, of the reaction below?
4Al (s) + 3MnO2 (s) → 2Al2O3 (s) + 3Mn (s)
A. −1670 + 520
B. (−1670) + 3(520)
C. 2(−1670) + 3(−520)
D. 2(−1670) + 3(520)
-
19N.2.hl.TZ0.6a(ii):
Copper(II) chloride is used as a catalyst in the production of chlorine from hydrogen chloride.
4HCl (g) + O2 (g) → 2Cl2 (g) + 2H2O (g)
Calculate the standard enthalpy change, ΔHθ, in kJ, for this reaction, using section 12 of the data booklet.
-
19N.2.sl.TZ0.3c(ii):
Determine the enthalpy of combustion of the organic product in (b), in kJ mol−1, using data from section 11 of the data booklet.
-
16N.3.sl.TZ0.6c:
(i) Suggest why incomplete combustion of plastic, such as polyvinyl chloride, is common in industrial and house fires.
(ii) Phthalate plasticizers such as DEHP, shown below, are frequently used in polyvinyl chloride.
With reference to bonding, suggest a reason why many adults have measurable levels of phthalates in their bodies.
-
16N.1.sl.TZ0.14:
In which reaction do the reactants have a lower potential energy than the products?
A. CH4(g) + 2O2(g) → CO2(g) + 2H2O(g)
B. HBr(g) → H(g) + Br(g)
C. Na+(g) + Cl-(g) → NaCl(s)
D. NaOH(aq) + HCl(aq) → NaCl(aq) + H2O(l) -
17M.3.sl.TZ1.15c:
Explain, in terms of the molecular structure, the critical difference in properties that makes biodiesel a more suitable liquid fuel than vegetable oil.
-
17N.1.sl.TZ0.15:
What is the enthalpy change, in kJ, of the following reaction?
3H2 (g) + N2 (g) 2NH3 (g)
A. (6 × 391) − [(3 × 436) + 945]
B. (3 × 391) − (436 + 945)
C. −[(3 × 436) + 945] + (3 × 391)
D. −(6 × 391) + [(3 × 436) + 945]
-
17N.2.sl.TZ0.1d.ii:
Calculate the enthalpy change, ΔH, in kJ mol–1, for the reaction between ethanoic acid and sodium hydroxide.
- 21M.2.hl.TZ1.7b: Explain why there are frequencies of UV light that will dissociate O3 but not O2.
-
18M.2.hl.TZ1.3c.iii:
Explain, giving two reasons, the difference in the values for (c)(i) and (ii). If you did not obtain answers, use −475 kJ for (i) and −600 kJ for (ii).
-
18M.2.sl.TZ1.3b.i:
Under certain conditions, ethyne can be converted to benzene.
Determine the standard enthalpy change, ΔHϴ, for the reaction stated, using section 11 of the data booklet.
3C2H2(g) → C6H6(g)
-
18M.2.sl.TZ2.4b.iii:
Outline why the value of enthalpy of reaction calculated from bond enthalpies is less accurate.
-
18N.2.hl.TZ0.1b.i:
The reaction was carried out in a calorimeter. The maximum temperature rise of the solution was 7.5 °C.
Calculate the enthalpy change, ΔH, of the reaction, in kJ, assuming that all the heat released was absorbed by the solution. Use sections 1 and 2 of the data booklet.
-
18N.2.hl.TZ0.1b.ii:
State another assumption you made in (b)(i).
- 22M.1.sl.TZ2.15: Which statement is correct about identical pieces of magnesium added to two solutions, X and Y,...
-
22M.1.hl.TZ2.16:
Which equation represents the bond enthalpy for H–Br in hydrogen bromide?
A. HBr (g) → H+ (g) + Br− (g)
B. HBr (g) → H (g) + Br (g)
C. HBr (g) → H2 (g) + Br2 (l)
D. HBr (g) → H2 (g) + Br2 (g)
-
22M.2.hl.TZ1.3b(i):
Determine the enthalpy change, ΔH, for the Haber–Bosch process, in kJ. Use Section 11 of the data booklet.
-
19M.2.hl.TZ2.1c(iv):
The IR spectrum and low resolution 1H NMR spectrum of the actual product formed are shown.
Deduce whether the product is A or B, using evidence from these spectra together with sections 26 and 27 of the data booklet.
Identity of product:
One piece of evidence from IR:
One piece of evidence from 1H NMR:
-
19M.1.hl.TZ2.14:
Methane undergoes incomplete combustion.
2CH4 (g) + 3O2 (g) → 2CO (g) + 4H2O (g)
What is the enthalpy change, in kJ, using the bond enthalpy data given below?
A. [2(1077) + 4(463)] − [2(414) + 3(498)]
B. [2(414) + 3(498)] − [2(1077) + 4(463)]
C. [8(414) + 3(498)] − [2(1077) + 8(463)]
D. [2(1077) + 8(463)] − [8(414) + 3(498)]
-
19M.3.sl.TZ1.2a(i):
Estimate the time at which the powdered zinc was placed in the beaker.
-
19N.2.hl.TZ0.6a(iii):
The diagram shows the Maxwell–Boltzmann distribution and potential energy profile for the reaction without a catalyst.
Annotate both charts to show the activation energy for the catalysed reaction, using the label Ea (cat).
-
19N.1.sl.TZ0.16:
Which equation represents the N–H bond enthalpy in NH3?
A. NH3 (g) → N (g) + 3H (g)
B. NH3 (g) → N (g) + H (g)
C. NH3 (g) → N2 (g) + H2 (g)
D. NH3 (g) → •NH2 (g) + •H (g)
-
16N.1.sl.TZ0.15:
5.35g of solid ammonium chloride, NH4Cl(s), was added to water to form 25.0g of solution. The maximum decrease in temperature was 14 K. What is the enthalpy change, in kJmol-1, for this reaction? (Molar mass of NH4Cl = 53.5gmol-1; the specific heat capacity of the solution is 4.18 Jg-1K-1)
A.
B.
C.
D.
-
20N.2.hl.TZ0.3a:
Determine the standard enthalpy change, , for this reaction, using section 11 of the data booklet.
- 17M.1.sl.TZ1.14: Which expression gives the enthalpy change, ΔH, for the thermal decomposition of calcium...
-
17M.2.sl.TZ1.4e:
Hydrazine has been used as a rocket fuel. The propulsion reaction occurs in several stages but the overall reaction is:
N2H4(l) → N2(g) + 2H2(g)
Suggest why this fuel is suitable for use at high altitudes.
-
17M.2.sl.TZ1.4f:
Determine the enthalpy change of reaction, ΔH, in kJ, when 1.00 mol of gaseous hydrazine decomposes to its elements. Use bond enthalpy values in section 11 of the data booklet.
N2H4(g) → N2(g) + 2H2(g)
-
17M.3.sl.TZ1.5f:
Outline why the thermochemical method would not be appropriate for 0.001 moldm−3 hydrochloric acid and aqueous sodium hydroxide of a similar concentration.
-
17M.2.sl.TZ2.8a.i:
Calculate the enthalpy change, in kJ, for the spray reaction, using the data below.
- 17N.2.hl.TZ0.1e: Suggest why the enthalpy change of neutralization of CH3COOH is less negative than that of HCl.
-
21M.1.sl.TZ1.15:
What is the enthalpy change of the reaction, in kJ?
2C (graphite) + O2 (g) → 2CO (g)
A. −394 − 283
B. 2(−394) + 2(−283)
C. −394 + 283
D. 2(−394) + 2(283)
- 21M.1.sl.TZ2.13: Which describes an exothermic reaction?
- 21M.1.sl.TZ2.15: Which is the enthalpy change of reaction, ΔH?
-
18M.2.hl.TZ1.3c.ii:
Determine the standard enthalpy change, ΔHΘ, for the following similar reaction, using ΔHf values in section 12 of the data booklet.
3C2H2(g) → C6H6(l)
- 18M.1.sl.TZ1.15: Which statement is correct? A. In an exothermic reaction, the products have more energy than...
- 18M.1.sl.TZ2.13: Which describes the reaction shown in the potential energy profile? A. The reaction is...
-
18M.1.sl.TZ2.15:
Two 100 cm3 aqueous solutions, one containing 0.010 mol NaOH and the other 0.010 mol HCl, are at the same temperature.
When the two solutions are mixed the temperature rises by y °C.
Assume the density of the final solution is 1.00 g cm−3.
Specific heat capacity of water = 4.18 J g−1 K−1
What is the enthalpy change of neutralization in kJ mol−1?
A.
B.
C.
D.
-
21N.2.sl.TZ0.3c(i):
Calculate the standard enthalpy change (ΔH⦵) for the forward reaction in kJ mol−1.
ΔH⦵f PCl3 (g) = −306.4 kJ mol−1
ΔH⦵f PCl5 (g) = −398.9 kJ mol−1
-
21N.2.sl.TZ0.7a:
Determine the molar enthalpy of combustion of an alkane if 8.75 × 10−4 moles are burned, raising the temperature of 20.0 g of water by 57.3 °C.
-
22M.1.sl.TZ1.13:
The energy from burning 0.250 g of ethanol causes the temperature of 150 cm3 of water to rise by 10.5 °C. What is the enthalpy of combustion of ethanol, in kJ mol–1?
Specific heat capacity of water: 4.18 J g–1 K–1.
A.
B.
C.
D.
-
19M.2.hl.TZ1.3g(i):
Determine the enthalpy change, ΔH, in kJ, for this reaction using data from the table and section 12 of the data booklet.
-
19M.1.hl.TZ1.15:
What is the enthalpy change of reaction for the following equation?
C2H4 (g) + H2 (g) → C2H6 (g)
C2H4 (g) + 3O2 (g) → 2CO2 (g) + 2H2O (l) ΔH = x
C2H6 (g) + O2 (g) → 2CO2 (g) + 3H2O (l) ΔH = y
H2 (g) + O2 (g) → H2O (l) ΔH = z
A. x + y + z
B. −x − y + z
C. x − y − z
D. x − y + z
-
20N.1.sl.TZ0.28:
A student obtained the following data to calculate , using .
What is the percentage uncertainty in the calculated value of ?
A.
B.
C.
D.
-
20N.1.hl.TZ0.13:
Which statement is correct?
A. bond dissociation occurs at a longer wavelength of light than bond dissociation.
B. bond dissociation occurs at a higher energy than bond dissociation.
C. bond lengths are shorter than bond lengths.
D. bond dissociation occurs at a higher frequency of light than bond dissociation.
-
20N.2.sl.TZ0.3a:
Determine the standard enthalpy change, , for this reaction, using section 11 of the data booklet.
- 17M.1.sl.TZ1.15: In which order does the oxygen–oxygen bond enthalpy increase? A. H2O2 < O2 < O3 B. ...
-
17M.3.sl.TZ1.5b:
Heat losses would make this method less accurate than the pH probe method. Outline why the thermometric method would always give a lower, not a higher, concentration.
-
17M.2.hl.TZ2.6b:
The overall equation for monochlorination of methane is:
CH4(g) + Cl2(g) → CH3Cl(g) + HCl(g)
Calculate the standard enthalpy change for the reaction, ΔH θ, using section 12 of the data booklet.
-
17M.2.hl.TZ2.8b.v:
State a technique other than a pH titration that can be used to detect the equivalence point.
-
17N.1.sl.TZ0.14:
The enthalpy changes for two reactions are given.
Br2 (l) + F2 (g) → 2BrF (g) ΔH = x kJ
Br2 (l) + 3F2 (g) → 2BrF3 (g) ΔH = y kJWhat is the enthalpy change for the following reaction?
BrF (g) + F2 (g) → BrF3 (g)
A. x – y
B. –x + y
C. (–x + y)
D. (x – y)
-
21M.1.sl.TZ2.14:
What is the heat change, in kJ, when 100.0 g of aluminium is heated from 19.0 °C to 32.0 °C?
Specific heat capacity of aluminium: 0.90 J g−1 K−1
A.
B.
C.
D.
-
21M.2.hl.TZ2.1b(iv):
Sketch an energy profile for the decomposition of calcium carbonate based on your answer to b(i), labelling the axes and activation energy, Ea.
-
18M.2.hl.TZ1.3c.i:
Under certain conditions, ethyne can be converted to benzene.
Determine the standard enthalpy change, ΔHΘ, for the reaction stated, using section 11 of the data booklet.
3C2H2(g) → C6H6(g)
-
18M.2.sl.TZ1.3b.iii:
Explain, giving two reasons, the difference in the values for (b)(i) and (ii). If you did not obtain answers, use −475 kJ for (i) and −600 kJ for (ii).
-
18M.2.sl.TZ2.4b.ii:
Determine the value of ΔHΘ, in kJ, for the reaction using the values in the table.
-
21N.1.sl.TZ0.14:
Which combustion reaction releases the least energy per mole of C3H8?
Approximate bond enthalpy / kJ mol−1
O=O 500
C=O 800
C≡O 1000
A. C3H8 (g) + 5O2 (g) → 3CO2 (g) + 4H2O (g)B. C3H8 (g) + O2 (g) → 2CO2 (g) + CO (g) + 4H2O (g)
C. C3H8 (g) + 4O2 (g) → CO2 (g) + 2CO (g) + 4H2O (g)
D. C3H8 (g) + O2 (g) → 3CO (g) + 4H2O (g)
Chemistry: Atoms First 2e, https://openstax.org/books/chemistry-atoms-first-2e/pages/9-4-strengths-of-ionic-andcovalent-bonds © 1999–2021, Rice University. Except where otherwise noted, textbooks on this site are licensed under a Creative Commons Attribution 4.0 International License.
(CC BY 4.0) https://creativecommons.org/licenses/ by/4.0/. - 21N.1.sl.TZ0.16: Which statement describes an endothermic reaction? A. The bonds broken are stronger than the...
- 22M.1.sl.TZ1.15: What is the correct interpretation of the following potential energy profile? A. Endothermic...
-
22M.2.sl.TZ1.2d(ii):
Calculate the enthalpy change, ΔH⦵, for the Haber–Bosch process, in kJ, using the following data.
.
-
22M.2.sl.TZ2.4e(i):
Calculate the enthalpy change of the reaction, ΔH, using section 11 of the data booklet.
- 22M.2.hl.TZ2.8f(ii): Draw and label an enthalpy level diagram for this reaction.
-
19M.2.hl.TZ2.3a(i):
Outline why ozone in the stratosphere is important.
-
19M.3.hl.TZ1.2b(ii):
To determine the enthalpy of reaction the experiment was carried out five times. The same volume and concentration of copper(II) sulfate was used but the mass of zinc was different each time. Suggest, with a reason, if zinc or copper(II) sulfate should be in excess for each trial.
-
19M.3.sl.TZ1.2b(iv):
Predict, giving a reason, how the final enthalpy of reaction calculated from this experiment would compare with the theoretical value.
-
19N.2.hl.TZ0.3c(ii):
Determine the enthalpy of combustion of this compound, in kJ mol−1, using data from section 11 of the data booklet.
- 19N.3.sl.TZ0.13a(ii): Explain why fusion is an exothermic process.
-
19N.2.sl.TZ0.5a(iii):
The diagram shows the Maxwell–Boltzmann distribution and potential energy profile for the reaction without a catalyst.
Annotate both charts to show the activation energy for the catalysed reaction, using the label Ea (cat).
-
16N.2.hl.TZ0.1b:
(i) Calculate ΔHθ, in kJ, for this similar reaction below using data from section 12 of the data booklet. of HOCH2CH2OH(l) is –454.8kJmol-1.
2CO (g) + 3H2 (g) HOCH2CH2OH (l)
(ii) Deduce why the answers to (a)(iii) and (b)(i) differ.
(iii) ΔSθ for the reaction in (b)(i) is –620.1JK-1. Comment on the decrease in entropy.
(iv) Calculate the value of ΔGθ, in kJ, for this reaction at 298 K using your answer to (b)(i). (If you did not obtain an answer to (b)(i), use –244.0 kJ, but this is not the correct value.)
(v) Comment on the statement that the reaction becomes less spontaneous as temperature is increased.
-
20N.1.sl.TZ0.13:
Which equation shows the enthalpy of formation, , of ethanol?
A.
B.
C.
D.
-
17M.2.sl.TZ2.8a.ii:
The energy released by the reaction of one mole of hydrogen peroxide with hydroquinone is used to heat 850 cm3 of water initially at 21.8°C. Determine the highest temperature reached by the water.
Specific heat capacity of water = 4.18 kJkg−1K−1.
(If you did not obtain an answer to part (i), use a value of 200.0 kJ for the energy released, although this is not the correct answer.)
-
17N.2.sl.TZ0.2b:
Explain why the melting points of the group 1 metals (Li → Cs) decrease down the group.
-
21M.2.hl.TZ1.3c:
Iron has a relatively small specific heat capacity; the temperature of a 50 g sample rises by 44.4°C when it absorbs 1 kJ of heat energy.
Determine the specific heat capacity of iron, in J g−1 K−1. Use section 1 of the data booklet.
-
21M.2.hl.TZ2.1b(i):
Calculate the enthalpy change of reaction, ΔH, in kJ, for the decomposition of calcium carbonate.
-
18M.1.sl.TZ1.14:
What is the enthalpy of combustion of butane in kJ mol−1?
2C4H10(g) + 13O2(g) → 8CO2(g) + 10H2O(l)
A. 4x + 5y − z
B. 4x + 5y + z
C. 8x + 10y − 2z
D. 8x + 5y + 2z
-
18M.2.sl.TZ1.3b.ii:
Determine the standard enthalpy change, ΔHΘ, for the following similar reaction, using ΔHf values in section 12 of the data booklet.
3C2H2(g) → C6H6(l)
-
18M.2.hl.TZ2.5b.i:
Outline why no value is listed for H2(g).
-
18M.2.hl.TZ2.5b.ii:
Determine the value of ΔHΘ, in kJ, for the reaction using the values in the table.
-
21N.2.hl.TZ0.3c(i):
Calculate the standard enthalpy change (ΔH⦵) for the forward reaction in kJ mol−1.
ΔH⦵f PCl3 (g) = −306.4 kJ mol−1
ΔH⦵f PCl5 (g) = −398.9 kJ mol−1
-
22M.1.sl.TZ2.14:
Which combination of ΔH1, ΔH2, and ΔH3 would give the enthalpy of the reaction?
CS2 (l) + 3O2 (g) → CO2 (g) + 2SO2 (g)
ΔH1 C (s) + O2 (g) → CO2 (g)
ΔH2 S (s) + O2 (g) → SO2 (g)
ΔH3 C (s) + 2S (s) → CS2 (l)A. ΔH = ΔH1 + ΔH2 + ΔH3
B. ΔH = ΔH1 + ΔH2 − ΔH3
C. ΔH = ΔH1 + 2(ΔH2) + ΔH3
D. ΔH = ΔH1 + 2(ΔH2) − ΔH3
-
22M.2.sl.TZ2.4e(ii):
Draw and label an enthalpy level diagram for this reaction.
-
19M.2.hl.TZ1.3g(ii):
Outline why bond enthalpy values are not valid in calculations such as that in (g)(i).
-
19M.2.hl.TZ1.3h:
An allotrope of molecular oxygen is ozone. Compare, giving a reason, the bond enthalpies of the O to O bonds in O2 and O3.
-
19M.3.hl.TZ1.2b(i):
The maximum temperature used to calculate the enthalpy of reaction was chosen at a point on the extrapolated (dotted) line.
State the maximum temperature which should be used and outline one assumption made in choosing this temperature on the extrapolated line.
Maximum temperature:
Assumption:
-
19M.2.sl.TZ1.3c(i):
Determine the enthalpy change, ΔH, in kJ, for this reaction using data from the table and section 12 of the data booklet.
-
19M.2.sl.TZ1.3c(ii):
Outline why bond enthalpy values are not valid in calculations such as that in (c)(i).
-
19M.1.sl.TZ1.15:
Which is correct for the reaction?
2Al (s) + 6HCl (aq) → 2AlCl3 (aq) + 3H2 (g) ΔH = −1049 kJ
A. Reactants are less stable than products and the reaction is endothermic.
B. Reactants are more stable than products and the reaction is endothermic.
C. Reactants are more stable than products and the reaction is exothermic.
D. Reactants are less stable than products and the reaction is exothermic.
- 16N.1.sl.TZ0.10: The C=N bond has a bond length of 130 pm and an average bond enthalpy of 615kJmol-1. Which values...
-
20N.1.sl.TZ0.15:
What is the bond enthalpy, in , in the molecule?
A.
B.
C.
D.
-
17N.2.hl.TZ0.5a:
Calculate the standard enthalpy change for this reaction using the following data.
- 21M.1.sl.TZ1.13: When sodium carbonate powder is added to ethanoic acid, the beaker becomes cooler. Possible...
-
21M.2.hl.TZ1.4d(ii):
State one reason why you would expect the value of ΔH calculated from the values, given in section 12 of data booklet, to differ from your answer to (d)(i).
- 21M.2.hl.TZ1.7a(ii): Discuss the relative length of the two O−O bonds in ozone.
-
21M.2.sl.TZ2.4b:
Determine the change in enthalpy, ΔH, for the combustion of but-2-ene, using section 11 of the data booklet.
CH3CH=CHCH3 (g) + 6O2 (g) → 4CO2 (g) + 4H2O (g)
-
21M.2.hl.TZ2.4b:
Determine the change in enthalpy, ΔH, for the combustion of but-2-ene, using section 11 of the data booklet.
CH3CH=CHCH3 (g) + 6O2 (g) → 4CO2 (g) + 4H2O (g)
-
18M.2.hl.TZ2.5a:
Hydrogen gas can be formed industrially by the reaction of natural gas with steam.
CH4(g) + H2O(g) → 3H2(g) + CO(g)
Determine the enthalpy change, ΔH, for the reaction, in kJ, using section 11 of the data booklet.
Bond enthalpy for C≡O: 1077 kJ mol−1
-
18N.1.sl.TZ0.14:
Which is correct when Ba(OH)2 reacts with NH4Cl?
Ba(OH)2 (s) + 2NH4Cl (s) → BaCl2 (aq) + 2NH3 (g) + 2H2O (l) ΔHΘ = +164 kJ mol−1
-
22M.2.sl.TZ1.2d(i):
Determine the enthalpy change, ΔH, for the Haber–Bosch process, in kJ. Use Section 11 of the data booklet.
- 22M.2.sl.TZ2.1d: Describe two observations that indicate the reaction of lithium with water is exothermic.
-
19M.3.sl.TZ1.2b(iii):
The formula q = mcΔT was used to calculate the energy released. The values used in the calculation were m = 25.00 g, c = 4.18 J g−1 K−1.
State an assumption made when using these values for m and c.
-
19M.2.sl.TZ2.1c(iv):
The enthalpy change for the reaction to produce B is −213 kJ. Predict, giving a reason, which product is the most stable.
-
19M.3.sl.TZ1.2b(ii):
To determine the enthalpy of reaction the experiment was carried out five times. The same volume and concentration of copper(II) sulfate was used but the mass of zinc was different each time. Suggest, with a reason, if zinc or copper(II) sulfate should be in excess for each trial.
-
19M.3.sl.TZ1.2a(ii):
State what point Y on the graph represents.
-
19N.2.sl.TZ0.5a(ii):
Copper(II) chloride is used as a catalyst in the production of chlorine from hydrogen chloride.
4HCl (g) + O2 (g) → 2Cl2 (g) + 2H2O (g)
Calculate the standard enthalpy change, ΔHθ, in kJ, for this reaction, using section 12 of the data booklet.
Sub sections and their related questions
5.1 Measuring energy changes
-
16N.1.sl.TZ0.1:
Which change of state is exothermic?
A. CO2(s) → CO2(g)
B. H2O(l) → H2O(g)
C. NH3(g) → NH3(l)
D. Fe(s) → Fe(l) -
16N.1.sl.TZ0.15:
5.35g of solid ammonium chloride, NH4Cl(s), was added to water to form 25.0g of solution. The maximum decrease in temperature was 14 K. What is the enthalpy change, in kJmol-1, for this reaction? (Molar mass of NH4Cl = 53.5gmol-1; the specific heat capacity of the solution is 4.18 Jg-1K-1)
A.
B.
C.
D.
-
17M.1.sl.TZ1.13:
Which expression gives the mass, in g, of ethanol required to produce 683.5 kJ of heat upon complete combustion?
(Mr for ethanol = 46.0, )
A.
B.
C.
D.
-
17M.2.sl.TZ1.4e:
Hydrazine has been used as a rocket fuel. The propulsion reaction occurs in several stages but the overall reaction is:
N2H4(l) → N2(g) + 2H2(g)
Suggest why this fuel is suitable for use at high altitudes.
-
17M.2.sl.TZ1.4f:
Determine the enthalpy change of reaction, ΔH, in kJ, when 1.00 mol of gaseous hydrazine decomposes to its elements. Use bond enthalpy values in section 11 of the data booklet.
N2H4(g) → N2(g) + 2H2(g)
-
17M.3.sl.TZ1.5a:
Explain how the concentration may be calculated in this way.
-
17M.3.sl.TZ1.5b:
Heat losses would make this method less accurate than the pH probe method. Outline why the thermometric method would always give a lower, not a higher, concentration.
-
17M.3.sl.TZ1.5c:
Suggest how heat loss could be reduced.
-
17M.3.sl.TZ1.5d:
State one other assumption that is usually made in the calculation of the heat produced.
-
17M.3.sl.TZ1.5f:
Outline why the thermochemical method would not be appropriate for 0.001 moldm−3 hydrochloric acid and aqueous sodium hydroxide of a similar concentration.
-
17M.3.sl.TZ1.15c:
Explain, in terms of the molecular structure, the critical difference in properties that makes biodiesel a more suitable liquid fuel than vegetable oil.
-
17M.2.sl.TZ2.8a.ii:
The energy released by the reaction of one mole of hydrogen peroxide with hydroquinone is used to heat 850 cm3 of water initially at 21.8°C. Determine the highest temperature reached by the water.
Specific heat capacity of water = 4.18 kJkg−1K−1.
(If you did not obtain an answer to part (i), use a value of 200.0 kJ for the energy released, although this is not the correct answer.)
-
17M.2.hl.TZ2.8b.v:
State a technique other than a pH titration that can be used to detect the equivalence point.
-
17N.1.sl.TZ0.13:
Which statement is correct for this reaction?
Fe2O3 (s) + 3CO (g) → 2Fe (s) + 3CO2 (g) ΔH = −26.6 kJ
A. 13.3 kJ are released for every mole of Fe produced.
B. 26.6 kJ are absorbed for every mole of Fe produced.
C. 53.2 kJ are released for every mole of Fe produced.
D. 26.6 kJ are released for every mole of Fe produced.
-
17N.1.hl.TZ0.17:
The combustion of glucose is exothermic and occurs according to the following equation:
C6H12O6 (s) + 6O2 (g) → 6CO2 (g) + 6H2O (g)
Which is correct for this reaction?
- 17N.1.hl.TZ0.19: The enthalpy change for the dissolution of NH4NO3 is +26 kJ mol–1 at 25 °C. Which statement...
-
17N.2.sl.TZ0.1d.i:
Determine the heat change, q, in kJ, for the neutralization reaction between ethanoic acid and sodium hydroxide.
Assume the specific heat capacities of the solutions and their densities are those of water.
-
17N.2.sl.TZ0.1d.ii:
Calculate the enthalpy change, ΔH, in kJ mol–1, for the reaction between ethanoic acid and sodium hydroxide.
-
17N.2.sl.TZ0.2b:
Explain why the melting points of the group 1 metals (Li → Cs) decrease down the group.
-
17N.2.hl.TZ0.3b:
Explain why the melting points of the group 1 metals (Li → Cs) decrease down the group whereas the melting points of the group 17 elements (F → I) increase down the group.
- 18M.1.sl.TZ1.13: The enthalpy of combustion of ethanol is determined by heating a known mass of tap water in a...
-
18M.1.sl.TZ2.15:
Two 100 cm3 aqueous solutions, one containing 0.010 mol NaOH and the other 0.010 mol HCl, are at the same temperature.
When the two solutions are mixed the temperature rises by y °C.
Assume the density of the final solution is 1.00 g cm−3.
Specific heat capacity of water = 4.18 J g−1 K−1
What is the enthalpy change of neutralization in kJ mol−1?
A.
B.
C.
D.
-
18N.1.sl.TZ0.14:
Which is correct when Ba(OH)2 reacts with NH4Cl?
Ba(OH)2 (s) + 2NH4Cl (s) → BaCl2 (aq) + 2NH3 (g) + 2H2O (l) ΔHΘ = +164 kJ mol−1
-
18N.2.sl.TZ0.1b.i:
The reaction was carried out in a calorimeter. The maximum temperature rise of the solution was 7.5 °C.
Calculate the enthalpy change, ΔH, of the reaction, in kJ, assuming that all the heat released was absorbed by the solution. Use sections 1 and 2 of the data booklet.
-
18N.2.sl.TZ0.1b.ii:
State another assumption you made in (b)(i).
-
18N.2.hl.TZ0.1b.i:
The reaction was carried out in a calorimeter. The maximum temperature rise of the solution was 7.5 °C.
Calculate the enthalpy change, ΔH, of the reaction, in kJ, assuming that all the heat released was absorbed by the solution. Use sections 1 and 2 of the data booklet.
-
18N.2.hl.TZ0.1b.ii:
State another assumption you made in (b)(i).
-
18N.2.sl.TZ0.7b:
Calculate the standard enthalpy change, ΔHΘ, of step 2 using section 13 of the data booklet.
-
19M.3.hl.TZ1.2a(ii):
State what point Y on the graph represents.
-
19M.3.hl.TZ1.2b(i):
The maximum temperature used to calculate the enthalpy of reaction was chosen at a point on the extrapolated (dotted) line.
State the maximum temperature which should be used and outline one assumption made in choosing this temperature on the extrapolated line.
Maximum temperature:
Assumption:
-
19M.3.hl.TZ1.2b(ii):
To determine the enthalpy of reaction the experiment was carried out five times. The same volume and concentration of copper(II) sulfate was used but the mass of zinc was different each time. Suggest, with a reason, if zinc or copper(II) sulfate should be in excess for each trial.
-
19M.3.hl.TZ1.2b(iii):
The formula q = mcΔT was used to calculate the energy released. The values used in the calculation were m = 25.00 g, c = 4.18 J g−1 K−1.
State an assumption made when using these values for m and c.
-
19M.3.hl.TZ1.2b(iv):
Predict, giving a reason, how the final enthalpy of reaction calculated from this experiment would compare with the theoretical value.
- 19M.1.hl.TZ1.14: When equal masses of X and Y absorb the same amount of energy, their temperatures rise by 5 °C...
-
19M.3.sl.TZ1.2a(i):
Estimate the time at which the powdered zinc was placed in the beaker.
-
19M.3.sl.TZ1.2a(ii):
State what point Y on the graph represents.
-
19M.3.sl.TZ1.2b(i):
The maximum temperature used to calculate the enthalpy of reaction was chosen at a point on the extrapolated (dotted) line.
State the maximum temperature which should be used and outline one assumption made in choosing this temperature on the extrapolated line.
Maximum temperature:
Assumption:
-
19M.3.sl.TZ1.2b(ii):
To determine the enthalpy of reaction the experiment was carried out five times. The same volume and concentration of copper(II) sulfate was used but the mass of zinc was different each time. Suggest, with a reason, if zinc or copper(II) sulfate should be in excess for each trial.
-
19M.3.sl.TZ1.2b(iii):
The formula q = mcΔT was used to calculate the energy released. The values used in the calculation were m = 25.00 g, c = 4.18 J g−1 K−1.
State an assumption made when using these values for m and c.
-
19M.3.sl.TZ1.2b(iv):
Predict, giving a reason, how the final enthalpy of reaction calculated from this experiment would compare with the theoretical value.
- 19M.1.sl.TZ1.13: When equal masses of X and Y absorb the same amount of energy, their temperatures rise by 5 °C...
- 19N.3.sl.TZ0.13a(ii): Explain why fusion is an exothermic process.
- 19N.3.hl.TZ0.18a(ii): Explain why fusion is an exothermic process.
-
19N.1.sl.TZ0.13:
What is the enthalpy of combustion, ΔHc, of ethanol in kJ mol−1?
Maximum temperature of water: 30.0°C
Initial temperature of water: 20.0°C
Mass of water in beaker: 100.0 g
Loss in mass of ethanol: 0.230 g
Mr (ethanol): 46.08
Specific heat capacity of water: 4.18 J g−1 K−1
q = mcΔTA.
B.
C.
D.
- 19N.1.sl.TZ0.14: Which quantity is likely to be the most inaccurate due to the sources of error in this...
-
20N.1.sl.TZ0.28:
A student obtained the following data to calculate , using .
What is the percentage uncertainty in the calculated value of ?
A.
B.
C.
D.
-
21M.1.sl.TZ1.14:
What is the enthalpy change, in J, when 5 g of water is heated from 10°C to 18°C?
Specific heat capacity of water: 4.18 kJ kg−1 K−1
A. 5 × 4.18 × 8
B. 5 × 10−3 × 4.18 × 8
C. 5 × 4.18 × (273 + 8)
D. 5 × 10−3 × 4.18 × (273 + 8)
- 21M.1.sl.TZ1.28: The enthalpy of combustion of a fuel was determined using the calorimeter shown. The final result...
-
21M.1.sl.TZ2.14:
What is the heat change, in kJ, when 100.0 g of aluminium is heated from 19.0 °C to 32.0 °C?
Specific heat capacity of aluminium: 0.90 J g−1 K−1
A.
B.
C.
D.
-
21M.2.sl.TZ1.3c:
Iron has a relatively small specific heat capacity; the temperature of a 50 g sample rises by 44.4°C when it absorbs 1 kJ of heat energy.
Determine the specific heat capacity of iron, in J g−1 K−1. Use section 1 of the data booklet.
-
21M.2.hl.TZ1.3c:
Iron has a relatively small specific heat capacity; the temperature of a 50 g sample rises by 44.4°C when it absorbs 1 kJ of heat energy.
Determine the specific heat capacity of iron, in J g−1 K−1. Use section 1 of the data booklet.
- 21N.1.sl.TZ0.16: Which statement describes an endothermic reaction? A. The bonds broken are stronger than the...
-
21N.2.sl.TZ0.7a:
Determine the molar enthalpy of combustion of an alkane if 8.75 × 10−4 moles are burned, raising the temperature of 20.0 g of water by 57.3 °C.
-
22M.1.sl.TZ1.13:
The energy from burning 0.250 g of ethanol causes the temperature of 150 cm3 of water to rise by 10.5 °C. What is the enthalpy of combustion of ethanol, in kJ mol–1?
Specific heat capacity of water: 4.18 J g–1 K–1.
A.
B.
C.
D.
- 22M.1.sl.TZ2.13: What is correct about energy changes during bond breaking and bond formation?
-
22M.1.sl.TZ2.14:
Which combination of ΔH1, ΔH2, and ΔH3 would give the enthalpy of the reaction?
CS2 (l) + 3O2 (g) → CO2 (g) + 2SO2 (g)
ΔH1 C (s) + O2 (g) → CO2 (g)
ΔH2 S (s) + O2 (g) → SO2 (g)
ΔH3 C (s) + 2S (s) → CS2 (l)A. ΔH = ΔH1 + ΔH2 + ΔH3
B. ΔH = ΔH1 + ΔH2 − ΔH3
C. ΔH = ΔH1 + 2(ΔH2) + ΔH3
D. ΔH = ΔH1 + 2(ΔH2) − ΔH3
- 22M.1.sl.TZ2.15: Which statement is correct about identical pieces of magnesium added to two solutions, X and Y,...
- 22M.2.sl.TZ2.1d: Describe two observations that indicate the reaction of lithium with water is exothermic.
5.2 Hess’s Law
-
16N.1.sl.TZ0.13:
Hydrazine reacts with oxygen.
N2H4(l) + O2(g) → N2(g) + 2H2O(l) ΔHθ = -623 kJ
What is the standard enthalpy of formation of N2H4(l) in kJ? The standard enthalpy of formation of H2O(l) is -286 kJ.
A. -623 - 286
B. -623 + 572
C. -572 + 623
D. -286 + 623 - 17M.1.sl.TZ1.14: Which expression gives the enthalpy change, ΔH, for the thermal decomposition of calcium...
-
17M.2.sl.TZ1.4g:
The standard enthalpy of formation of N2H4(l) is +50.6 kJmol−1. Calculate the enthalpy of vaporization, ΔHvap, of hydrazine in kJmol−1.
N2H4(l) → N2H4(g)
(If you did not get an answer to (f), use −85 kJ but this is not the correct answer.)
-
17M.1.sl.TZ2.14:
Why is the value of the enthalpy change of this reaction calculated from bond enthalpy data less accurate than that calculated from standard enthalpies of formation?
2C2H6(g) + 7O2(g) → 4CO2(g) + 6H2O(g)
A. All the reactants and products are gases.
B. Bond enthalpy data are average values for many compounds.
C. Elements do not have standard enthalpy of formation.
D. Standard enthalpies of formation are per mole.
-
17M.2.sl.TZ2.8a.i:
Calculate the enthalpy change, in kJ, for the spray reaction, using the data below.
-
17M.2.hl.TZ2.6b:
The overall equation for monochlorination of methane is:
CH4(g) + Cl2(g) → CH3Cl(g) + HCl(g)
Calculate the standard enthalpy change for the reaction, ΔH θ, using section 12 of the data booklet.
-
17N.1.sl.TZ0.14:
The enthalpy changes for two reactions are given.
Br2 (l) + F2 (g) → 2BrF (g) ΔH = x kJ
Br2 (l) + 3F2 (g) → 2BrF3 (g) ΔH = y kJWhat is the enthalpy change for the following reaction?
BrF (g) + F2 (g) → BrF3 (g)
A. x – y
B. –x + y
C. (–x + y)
D. (x – y)
-
17N.2.hl.TZ0.5a:
Calculate the standard enthalpy change for this reaction using the following data.
-
18M.2.hl.TZ1.3c.ii:
Determine the standard enthalpy change, ΔHΘ, for the following similar reaction, using ΔHf values in section 12 of the data booklet.
3C2H2(g) → C6H6(l)
-
18M.1.sl.TZ1.14:
What is the enthalpy of combustion of butane in kJ mol−1?
2C4H10(g) + 13O2(g) → 8CO2(g) + 10H2O(l)
A. 4x + 5y − z
B. 4x + 5y + z
C. 8x + 10y − 2z
D. 8x + 5y + 2z
-
18M.2.sl.TZ1.3b.ii:
Determine the standard enthalpy change, ΔHΘ, for the following similar reaction, using ΔHf values in section 12 of the data booklet.
3C2H2(g) → C6H6(l)
-
18M.1.sl.TZ2.14:
What is the enthalpy change of combustion of urea, (NH2)2CO, in kJ mol−1?
2(NH2)2CO(s) + 3O2(g) → 2CO2(g) + 2N2(g) + 4H2O(l)

A. 2 × (−333) −2 × (−394) −4 × (−286)
B. [2 × (−394) + 4 × (−286) −2 × (−333)]
C. 2 × (−394) + 4 × (−286) −2 × (−333)
D. [2 × (−333) −2 × (−394) −4 × (−286)]
-
18M.2.sl.TZ2.4b.i:
Outline why no value is listed for H2(g).
-
18M.2.sl.TZ2.4b.ii:
Determine the value of ΔHΘ, in kJ, for the reaction using the values in the table.
-
18M.2.hl.TZ2.5b.i:
Outline why no value is listed for H2(g).
-
18M.2.hl.TZ2.5b.ii:
Determine the value of ΔHΘ, in kJ, for the reaction using the values in the table.
-
18N.1.sl.TZ0.13:
Consider the following reactions:
Fe2O3 (s) + CO (g) → 2FeO (s) + CO2 (g) ΔHΘ = −3 kJ
Fe (s) + CO2 (g) → FeO (s) + CO (g) ΔHΘ = +11 kJ
What is the ΔHΘ value, in kJ, for the following reaction?
Fe2O3 (s) + 3CO (g) → 2Fe (s) + 3CO2 (g)
A. −25
B. −14
C. +8
D. +19
-
18N.2.sl.TZ0.7c:
Determine the standard enthalpy change, ΔHΘ, of step 1.
-
19M.2.hl.TZ1.3g(i):
Determine the enthalpy change, ΔH, in kJ, for this reaction using data from the table and section 12 of the data booklet.
-
19M.1.hl.TZ1.15:
What is the enthalpy change of reaction for the following equation?
C2H4 (g) + H2 (g) → C2H6 (g)
C2H4 (g) + 3O2 (g) → 2CO2 (g) + 2H2O (l) ΔH = x
C2H6 (g) + O2 (g) → 2CO2 (g) + 3H2O (l) ΔH = y
H2 (g) + O2 (g) → H2O (l) ΔH = z
A. x + y + z
B. −x − y + z
C. x − y − z
D. x − y + z
-
19M.2.sl.TZ1.3c(i):
Determine the enthalpy change, ΔH, in kJ, for this reaction using data from the table and section 12 of the data booklet.
- 19M.1.sl.TZ1.14: What is the enthalpy change of reaction for the following equation? A. x + y + z B. −x − y +...
-
19M.1.sl.TZ2.13:
Consider the following equations.
2Al (s) + O2 (g) → Al2O3 (s) ΔHƟ = −1670 kJ
Mn (s) + O2 (g) → MnO2 (s) ΔHƟ = −520 kJWhat is the standard enthalpy change, in kJ, of the reaction below?
4Al (s) + 3MnO2 (s) → 2Al2O3 (s) + 3Mn (s)
A. −1670 + 520
B. (−1670) + 3(520)
C. 2(−1670) + 3(−520)
D. 2(−1670) + 3(520)
-
19N.2.hl.TZ0.6a(ii):
Copper(II) chloride is used as a catalyst in the production of chlorine from hydrogen chloride.
4HCl (g) + O2 (g) → 2Cl2 (g) + 2H2O (g)
Calculate the standard enthalpy change, ΔHθ, in kJ, for this reaction, using section 12 of the data booklet.
-
19N.1.sl.TZ0.15:
What is the enthalpy change of the reaction?
C6H14 (l) → C2H4 (g) + C4H10 (g)
A. + 1411 + 2878 + 4163
B. + 1411 − 2878 − 4163
C. + 1411 + 2878 − 4163
D. − 1411 − 2878 + 4163
-
19N.2.sl.TZ0.5a(ii):
Copper(II) chloride is used as a catalyst in the production of chlorine from hydrogen chloride.
4HCl (g) + O2 (g) → 2Cl2 (g) + 2H2O (g)
Calculate the standard enthalpy change, ΔHθ, in kJ, for this reaction, using section 12 of the data booklet.
-
20N.1.sl.TZ0.13:
Which equation shows the enthalpy of formation, , of ethanol?
A.
B.
C.
D.
-
20N.1.sl.TZ0.14:
Which combination will give you the enthalpy change for the hydrogenation of ethene to ethane, ?
A.
B.
C.
D.
-
21M.1.sl.TZ1.15:
What is the enthalpy change of the reaction, in kJ?
2C (graphite) + O2 (g) → 2CO (g)
A. −394 − 283
B. 2(−394) + 2(−283)
C. −394 + 283
D. 2(−394) + 2(283)
-
21M.2.sl.TZ2.1b:
Thermodynamic data for the decomposition of calcium carbonate is given.
Calculate the enthalpy change of reaction, ΔH, in kJ, for the decomposition of calcium carbonate.
-
21M.2.hl.TZ2.1b(i):
Calculate the enthalpy change of reaction, ΔH, in kJ, for the decomposition of calcium carbonate.
-
21N.1.sl.TZ0.15:
Which equation represents the standard enthalpy of formation of lithium oxide?
A. 4Li (s) + O2 (g) → 2Li2O (s)B. 2Li (s) + O2 (g) → Li2O (s)
C. Li (s) + O2 (g) → Li2O (s)
D. Li (g) + O2 (g) → Li2O (g)
-
21N.2.sl.TZ0.3c(i):
Calculate the standard enthalpy change (ΔH⦵) for the forward reaction in kJ mol−1.
ΔH⦵f PCl3 (g) = −306.4 kJ mol−1
ΔH⦵f PCl5 (g) = −398.9 kJ mol−1
-
21N.2.hl.TZ0.3c(i):
Calculate the standard enthalpy change (ΔH⦵) for the forward reaction in kJ mol−1.
ΔH⦵f PCl3 (g) = −306.4 kJ mol−1
ΔH⦵f PCl5 (g) = −398.9 kJ mol−1
- 22M.1.sl.TZ1.14: What is the enthalpy change of the following reaction? CH2CHCH2CH3 + HBr → CH3CHBrCH2CH3 A. ...
-
22M.2.sl.TZ1.2d(ii):
Calculate the enthalpy change, ΔH⦵, for the Haber–Bosch process, in kJ, using the following data.
.
5.3 Bond enthalpies
- 16N.1.sl.TZ0.10: The C=N bond has a bond length of 130 pm and an average bond enthalpy of 615kJmol-1. Which values...
-
16N.1.sl.TZ0.14:
In which reaction do the reactants have a lower potential energy than the products?
A. CH4(g) + 2O2(g) → CO2(g) + 2H2O(g)
B. HBr(g) → H(g) + Br(g)
C. Na+(g) + Cl-(g) → NaCl(s)
D. NaOH(aq) + HCl(aq) → NaCl(aq) + H2O(l) -
16N.2.sl.TZ0.1a:
Ethane-1,2-diol can be formed according to the following reaction.
2CO (g) + 3H2 (g) HOCH2CH2OH (g)
(i) Deduce the equilibrium constant expression, Kc, for this reaction.
(ii) State how increasing the pressure of the reaction mixture at constant temperature will affect the position of equilibrium and the value of Kc.
Position of equilibrium:
Kc:
(iii) Calculate the enthalpy change, ΔHθ, in kJ, for this reaction using section 11 of the data booklet. The bond enthalpy of the carbon–oxygen bond in CO (g) is 1077kJmol-1.
(iv) The enthalpy change, ΔHθ, for the following similar reaction is –233.8 kJ.
2CO(g) + 3H2(g) HOCH2CH2OH (l)
Deduce why this value differs from your answer to (a)(iii).
-
16N.2.hl.TZ0.1b:
(i) Calculate ΔHθ, in kJ, for this similar reaction below using data from section 12 of the data booklet. of HOCH2CH2OH(l) is –454.8kJmol-1.
2CO (g) + 3H2 (g) HOCH2CH2OH (l)
(ii) Deduce why the answers to (a)(iii) and (b)(i) differ.
(iii) ΔSθ for the reaction in (b)(i) is –620.1JK-1. Comment on the decrease in entropy.
(iv) Calculate the value of ΔGθ, in kJ, for this reaction at 298 K using your answer to (b)(i). (If you did not obtain an answer to (b)(i), use –244.0 kJ, but this is not the correct value.)
(v) Comment on the statement that the reaction becomes less spontaneous as temperature is increased.
-
16N.3.sl.TZ0.6c:
(i) Suggest why incomplete combustion of plastic, such as polyvinyl chloride, is common in industrial and house fires.
(ii) Phthalate plasticizers such as DEHP, shown below, are frequently used in polyvinyl chloride.
With reference to bonding, suggest a reason why many adults have measurable levels of phthalates in their bodies.
- 17M.1.sl.TZ1.15: In which order does the oxygen–oxygen bond enthalpy increase? A. H2O2 < O2 < O3 B. ...
- 17M.1.sl.TZ2.13: What can be deduced from this reaction profile? A. The reactants are less stable than the...
- 17M.1.sl.TZ2.15: What can be deduced from the facts that ozone absorbs UV radiation in the region of 340 nm...
-
17M.2.sl.TZ2.5b:
Nitrogen dioxide and carbon monoxide react according to the following equation:
NO2(g) + CO(g) NO(g) + CO2(g) ΔH = –226 kJ
Calculate the activation energy for the reverse reaction.
-
17N.1.sl.TZ0.15:
What is the enthalpy change, in kJ, of the following reaction?
3H2 (g) + N2 (g) 2NH3 (g)
A. (6 × 391) − [(3 × 436) + 945]
B. (3 × 391) − (436 + 945)
C. −[(3 × 436) + 945] + (3 × 391)
D. −(6 × 391) + [(3 × 436) + 945]
- 17N.2.hl.TZ0.1e: Suggest why the enthalpy change of neutralization of CH3COOH is less negative than that of HCl.
-
18M.2.hl.TZ1.3c.i:
Under certain conditions, ethyne can be converted to benzene.
Determine the standard enthalpy change, ΔHΘ, for the reaction stated, using section 11 of the data booklet.
3C2H2(g) → C6H6(g)
-
18M.2.hl.TZ1.3c.iii:
Explain, giving two reasons, the difference in the values for (c)(i) and (ii). If you did not obtain answers, use −475 kJ for (i) and −600 kJ for (ii).
- 18M.1.sl.TZ1.15: Which statement is correct? A. In an exothermic reaction, the products have more energy than...
-
18M.2.sl.TZ1.3b.i:
Under certain conditions, ethyne can be converted to benzene.
Determine the standard enthalpy change, ΔHϴ, for the reaction stated, using section 11 of the data booklet.
3C2H2(g) → C6H6(g)
-
18M.2.sl.TZ1.3b.iii:
Explain, giving two reasons, the difference in the values for (b)(i) and (ii). If you did not obtain answers, use −475 kJ for (i) and −600 kJ for (ii).
- 18M.1.sl.TZ2.13: Which describes the reaction shown in the potential energy profile? A. The reaction is...
-
18M.2.sl.TZ2.4a:
Hydrogen gas can be formed industrially by the reaction of natural gas with steam.
CH4(g) + H2O(g) → 3H2(g) + CO(g)
Determine the enthalpy change, ΔH, for the reaction, in kJ, using section 11 of the data booklet.
Bond enthalpy for C≡O: 1077 kJ mol−1
-
18M.2.sl.TZ2.4b.iii:
Outline why the value of enthalpy of reaction calculated from bond enthalpies is less accurate.
-
18M.2.hl.TZ2.5a:
Hydrogen gas can be formed industrially by the reaction of natural gas with steam.
CH4(g) + H2O(g) → 3H2(g) + CO(g)
Determine the enthalpy change, ΔH, for the reaction, in kJ, using section 11 of the data booklet.
Bond enthalpy for C≡O: 1077 kJ mol−1
-
18N.1.sl.TZ0.15:
Consider the following reaction:
N2 (g) + 3H2 (g) 2NH3 (g)
Which calculation gives ΔHΘ, in kJ, for the forward reaction?
A. 2z − y − 3x
B. y + 3x − 2z
C. y + 3x − 6z
D. 6z − y − 3x
-
19M.2.hl.TZ1.3g(ii):
Outline why bond enthalpy values are not valid in calculations such as that in (g)(i).
-
19M.2.hl.TZ1.3h:
An allotrope of molecular oxygen is ozone. Compare, giving a reason, the bond enthalpies of the O to O bonds in O2 and O3.
-
19M.2.hl.TZ2.1c(iv):
The IR spectrum and low resolution 1H NMR spectrum of the actual product formed are shown.
Deduce whether the product is A or B, using evidence from these spectra together with sections 26 and 27 of the data booklet.
Identity of product:
One piece of evidence from IR:
One piece of evidence from 1H NMR:
-
19M.2.hl.TZ2.3a(i):
Outline why ozone in the stratosphere is important.
-
19M.1.hl.TZ2.14:
Methane undergoes incomplete combustion.
2CH4 (g) + 3O2 (g) → 2CO (g) + 4H2O (g)
What is the enthalpy change, in kJ, using the bond enthalpy data given below?
A. [2(1077) + 4(463)] − [2(414) + 3(498)]
B. [2(414) + 3(498)] − [2(1077) + 4(463)]
C. [8(414) + 3(498)] − [2(1077) + 8(463)]
D. [2(1077) + 8(463)] − [8(414) + 3(498)]
-
19M.2.sl.TZ1.3c(ii):
Outline why bond enthalpy values are not valid in calculations such as that in (c)(i).
-
19M.2.sl.TZ2.1c(iii):
Determine the enthalpy change for the reaction, in kJ, to produce A using section 11 of the data booklet.
-
19M.2.sl.TZ2.1c(iv):
The enthalpy change for the reaction to produce B is −213 kJ. Predict, giving a reason, which product is the most stable.
-
19M.2.sl.TZ2.3a:
Outline why ozone in the stratosphere is important.
-
19M.1.sl.TZ1.15:
Which is correct for the reaction?
2Al (s) + 6HCl (aq) → 2AlCl3 (aq) + 3H2 (g) ΔH = −1049 kJ
A. Reactants are less stable than products and the reaction is endothermic.
B. Reactants are more stable than products and the reaction is endothermic.
C. Reactants are more stable than products and the reaction is exothermic.
D. Reactants are less stable than products and the reaction is exothermic.
-
19M.1.sl.TZ2.14:
Methane undergoes incomplete combustion.
2CH4 (g) + 3O2 (g) → 2CO (g) + 4H2O (g)
What is the enthalpy change, in kJ, using the bond enthalpy data given below?
A. [2(1077) + 4(463)] − [2(414) + 3(498)]
B. [2(414) + 3(498)] − [2(1077) + 4(463)]
C. [8(414) + 3(498)] − [2(1077) + 8(463)]
D. [2(1077) + 8(463)] − [8(414) + 3(498)]
-
19N.2.hl.TZ0.3c(ii):
Determine the enthalpy of combustion of this compound, in kJ mol−1, using data from section 11 of the data booklet.
-
19N.2.hl.TZ0.6a(iii):
The diagram shows the Maxwell–Boltzmann distribution and potential energy profile for the reaction without a catalyst.
Annotate both charts to show the activation energy for the catalysed reaction, using the label Ea (cat).
-
19N.2.sl.TZ0.3c(ii):
Determine the enthalpy of combustion of the organic product in (b), in kJ mol−1, using data from section 11 of the data booklet.
-
19N.2.sl.TZ0.5a(iii):
The diagram shows the Maxwell–Boltzmann distribution and potential energy profile for the reaction without a catalyst.
Annotate both charts to show the activation energy for the catalysed reaction, using the label Ea (cat).
-
19N.1.sl.TZ0.16:
Which equation represents the N–H bond enthalpy in NH3?
A. NH3 (g) → N (g) + 3H (g)
B. NH3 (g) → N (g) + H (g)
C. NH3 (g) → N2 (g) + H2 (g)
D. NH3 (g) → •NH2 (g) + •H (g)
-
20N.1.sl.TZ0.15:
What is the bond enthalpy, in , in the molecule?
A.
B.
C.
D.
-
20N.1.hl.TZ0.13:
Which statement is correct?
A. bond dissociation occurs at a longer wavelength of light than bond dissociation.
B. bond dissociation occurs at a higher energy than bond dissociation.
C. bond lengths are shorter than bond lengths.
D. bond dissociation occurs at a higher frequency of light than bond dissociation.
-
20N.1.hl.TZ0.15:
Which statements about bond strength and activation energy are correct for this reaction?
-
20N.2.sl.TZ0.3a:
Determine the standard enthalpy change, , for this reaction, using section 11 of the data booklet.
-
20N.2.sl.TZ0.3b:
Calculate the standard enthalpy change, , for this reaction using section 12 of the data booklet.
-
20N.2.hl.TZ0.3a:
Determine the standard enthalpy change, , for this reaction, using section 11 of the data booklet.
-
20N.2.hl.TZ0.3b:
Calculate the standard enthalpy change, , for this reaction using section 12 of the data booklet.
- 21M.1.sl.TZ1.13: When sodium carbonate powder is added to ethanoic acid, the beaker becomes cooler. Possible...
- 21M.1.sl.TZ2.13: Which describes an exothermic reaction?
- 21M.1.sl.TZ2.15: Which is the enthalpy change of reaction, ΔH?
- 21M.1.hl.TZ2.15: The potential energy profile of a reaction is shown. What can be determined about stability...
-
21M.2.sl.TZ1.4d(i):
Determine the enthalpy change, ΔH, in kJ. Use section 11 of the data booklet.
Bond enthalpy of CO = 1077 kJ mol−1.
-
21M.2.hl.TZ1.4d(i):
Determine the enthalpy change, ΔH, in kJ. Use section 11 of the data booklet.
Bond enthalpy of CO = 1077 kJ mol−1.
-
21M.2.hl.TZ1.4d(ii):
State one reason why you would expect the value of ΔH calculated from the values, given in section 12 of data booklet, to differ from your answer to (d)(i).
- 21M.2.hl.TZ1.7a(ii): Discuss the relative length of the two O−O bonds in ozone.
- 21M.2.hl.TZ1.7b: Explain why there are frequencies of UV light that will dissociate O3 but not O2.
-
21M.2.hl.TZ1.7c:
Explain, using equations, how the presence of results in a chain reaction that decreases the concentration of ozone in the stratosphere.
- 21M.2.sl.TZ2.1c(i): The potential energy profile for a reaction is shown. Sketch a dotted line labelled “Catalysed”...
-
21M.2.sl.TZ2.4b:
Determine the change in enthalpy, ΔH, for the combustion of but-2-ene, using section 11 of the data booklet.
CH3CH=CHCH3 (g) + 6O2 (g) → 4CO2 (g) + 4H2O (g)
-
21M.2.hl.TZ2.1b(iv):
Sketch an energy profile for the decomposition of calcium carbonate based on your answer to b(i), labelling the axes and activation energy, Ea.
-
21M.2.hl.TZ2.4b:
Determine the change in enthalpy, ΔH, for the combustion of but-2-ene, using section 11 of the data booklet.
CH3CH=CHCH3 (g) + 6O2 (g) → 4CO2 (g) + 4H2O (g)
-
21N.1.sl.TZ0.14:
Which combustion reaction releases the least energy per mole of C3H8?
Approximate bond enthalpy / kJ mol−1
O=O 500
C=O 800
C≡O 1000
A. C3H8 (g) + 5O2 (g) → 3CO2 (g) + 4H2O (g)B. C3H8 (g) + O2 (g) → 2CO2 (g) + CO (g) + 4H2O (g)
C. C3H8 (g) + 4O2 (g) → CO2 (g) + 2CO (g) + 4H2O (g)
D. C3H8 (g) + O2 (g) → 3CO (g) + 4H2O (g)
Chemistry: Atoms First 2e, https://openstax.org/books/chemistry-atoms-first-2e/pages/9-4-strengths-of-ionic-andcovalent-bonds © 1999–2021, Rice University. Except where otherwise noted, textbooks on this site are licensed under a Creative Commons Attribution 4.0 International License.
(CC BY 4.0) https://creativecommons.org/licenses/ by/4.0/. - 21N.1.sl.TZ0.16: Which statement describes an endothermic reaction? A. The bonds broken are stronger than the...
- 21N.2.sl.TZ0.4a(iii): Suggest, with a reason, why 1-iodopentane reacts faster than 1-chloropentane under the same...
- 22M.1.sl.TZ1.15: What is the correct interpretation of the following potential energy profile? A. Endothermic...
-
22M.1.hl.TZ2.16:
Which equation represents the bond enthalpy for H–Br in hydrogen bromide?
A. HBr (g) → H+ (g) + Br− (g)
B. HBr (g) → H (g) + Br (g)
C. HBr (g) → H2 (g) + Br2 (l)
D. HBr (g) → H2 (g) + Br2 (g)
-
22M.2.sl.TZ1.2d(i):
Determine the enthalpy change, ΔH, for the Haber–Bosch process, in kJ. Use Section 11 of the data booklet.
-
22M.2.sl.TZ1.2d(iii):
Suggest why the values obtained in (d)(i) and (d)(ii) differ.
-
22M.2.hl.TZ1.3b(i):
Determine the enthalpy change, ΔH, for the Haber–Bosch process, in kJ. Use Section 11 of the data booklet.
-
22M.2.hl.TZ1.3b(ii):
Outline why the value obtained in (b)(i) might differ from a value calculated using ΔHf data.
-
22M.2.sl.TZ2.4e(i):
Calculate the enthalpy change of the reaction, ΔH, using section 11 of the data booklet.
-
22M.2.sl.TZ2.4e(ii):
Draw and label an enthalpy level diagram for this reaction.
-
22M.2.hl.TZ2.8f(i):
Calculate the enthalpy change of the reaction, ΔH, using section 11 of the data booklet.
- 22M.2.hl.TZ2.8f(ii): Draw and label an enthalpy level diagram for this reaction.
