| Date | May 2018 | Marks available | 1 | Reference code | 18M.1.sl.TZ2.14 |
| Level | SL | Paper | 1 | Time zone | TZ2 |
| Command term | Derive | Question number | 14 | Adapted from | N/A |
Question
What is the enthalpy change of combustion of urea, (NH2)2CO, in kJ mol−1?
2(NH2)2CO(s) + 3O2(g) → 2CO2(g) + 2N2(g) + 4H2O(l)
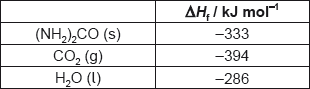
A. 2 × (−333) −2 × (−394) −4 × (−286)
B. [2 × (−394) + 4 × (−286) −2 × (−333)]
C. 2 × (−394) + 4 × (−286) −2 × (−333)
D. [2 × (−333) −2 × (−394) −4 × (−286)]
Markscheme
B
Examiners report
Syllabus sections
-
22M.2.sl.TZ1.2d(ii):
Calculate the enthalpy change, ΔH⦵, for the Haber–Bosch process, in kJ, using the following data.
.
- 22M.1.sl.TZ1.14: What is the enthalpy change of the following reaction? CH2CHCH2CH3 + HBr →...
- 17M.1.sl.TZ1.14: Which expression gives the enthalpy change, ΔH, for the thermal decomposition of calcium...
-
16N.1.sl.TZ0.13:
Hydrazine reacts with oxygen.
N2H4(l) + O2(g) → N2(g) + 2H2O(l) ΔHθ = -623 kJ
What is the standard enthalpy of formation of N2H4(l) in kJ? The standard enthalpy of formation of H2O(l) is -286 kJ.
A. -623 - 286
B. -623 + 572
C. -572 + 623
D. -286 + 623 -
17M.2.hl.TZ2.6b:
The overall equation for monochlorination of methane is:
CH4(g) + Cl2(g) → CH3Cl(g) + HCl(g)
Calculate the standard enthalpy change for the reaction, ΔH θ, using section 12 of the data booklet.
-
17N.1.sl.TZ0.14:
The enthalpy changes for two reactions are given.
Br2 (l) + F2 (g) → 2BrF (g) ΔH = x kJ
Br2 (l) + 3F2 (g) → 2BrF3 (g) ΔH = y kJWhat is the enthalpy change for the following reaction?
BrF (g) + F2 (g) → BrF3 (g)
A. x – y
B. –x + y
C. (–x + y)
D. (x – y)
-
18M.2.hl.TZ2.5b.i:
Outline why no value is listed for H2(g).
- 19M.1.sl.TZ1.14: What is the enthalpy change of reaction for the following equation? A. x + y + z B. −x −...
-
19M.1.hl.TZ1.15:
What is the enthalpy change of reaction for the following equation?
C2H4 (g) + H2 (g) → C2H6 (g)
C2H4 (g) + 3O2 (g) → 2CO2 (g) + 2H2O (l) ΔH = x
C2H6 (g) + O2 (g) → 2CO2 (g) + 3H2O (l) ΔH = y
H2 (g) + O2 (g) → H2O (l) ΔH = z
A. x + y + z
B. −x − y + z
C. x − y − z
D. x − y + z
-
17M.2.sl.TZ1.4g:
The standard enthalpy of formation of N2H4(l) is +50.6 kJmol−1. Calculate the enthalpy of vaporization, ΔHvap, of hydrazine in kJmol−1.
N2H4(l) → N2H4(g)
(If you did not get an answer to (f), use −85 kJ but this is not the correct answer.)
-
20N.1.sl.TZ0.13:
Which equation shows the enthalpy of formation, , of ethanol?
A.
B.
C.
D.
-
17N.2.hl.TZ0.5a:
Calculate the standard enthalpy change for this reaction using the following data.
-
18N.2.sl.TZ0.7c:
Determine the standard enthalpy change, ΔHΘ, of step 1.
-
17M.1.sl.TZ2.14:
Why is the value of the enthalpy change of this reaction calculated from bond enthalpy data less accurate than that calculated from standard enthalpies of formation?
2C2H6(g) + 7O2(g) → 4CO2(g) + 6H2O(g)
A. All the reactants and products are gases.
B. Bond enthalpy data are average values for many compounds.
C. Elements do not have standard enthalpy of formation.
D. Standard enthalpies of formation are per mole.
-
19M.2.hl.TZ1.3g(i):
Determine the enthalpy change, ΔH, in kJ, for this reaction using data from the table and section 12 of the data booklet.
-
18M.2.sl.TZ2.4b.ii:
Determine the value of ΔHΘ, in kJ, for the reaction using the values in the table.
-
18M.2.sl.TZ2.4b.i:
Outline why no value is listed for H2(g).
-
18N.1.sl.TZ0.13:
Consider the following reactions:
Fe2O3 (s) + CO (g) → 2FeO (s) + CO2 (g) ΔHΘ = −3 kJ
Fe (s) + CO2 (g) → FeO (s) + CO (g) ΔHΘ = +11 kJ
What is the ΔHΘ value, in kJ, for the following reaction?
Fe2O3 (s) + 3CO (g) → 2Fe (s) + 3CO2 (g)
A. −25
B. −14
C. +8
D. +19
-
19N.1.sl.TZ0.15:
What is the enthalpy change of the reaction?
C6H14 (l) → C2H4 (g) + C4H10 (g)
A. + 1411 + 2878 + 4163
B. + 1411 − 2878 − 4163
C. + 1411 + 2878 − 4163
D. − 1411 − 2878 + 4163
-
19M.2.sl.TZ1.3c(i):
Determine the enthalpy change, ΔH, in kJ, for this reaction using data from the table and section 12 of the data booklet.
-
18M.1.sl.TZ1.14:
What is the enthalpy of combustion of butane in kJ mol−1?
2C4H10(g) + 13O2(g) → 8CO2(g) + 10H2O(l)
A. 4x + 5y − z
B. 4x + 5y + z
C. 8x + 10y − 2z
D. 8x + 5y + 2z
-
19N.2.sl.TZ0.5a(ii):
Copper(II) chloride is used as a catalyst in the production of chlorine from hydrogen chloride.
4HCl (g) + O2 (g) → 2Cl2 (g) + 2H2O (g)
Calculate the standard enthalpy change, ΔHθ, in kJ, for this reaction, using section 12 of the data booklet.
-
21M.1.sl.TZ1.15:
What is the enthalpy change of the reaction, in kJ?
2C (graphite) + O2 (g) → 2CO (g)
A. −394 − 283
B. 2(−394) + 2(−283)
C. −394 + 283
D. 2(−394) + 2(283)
-
18M.2.hl.TZ1.3c.ii:
Determine the standard enthalpy change, ΔHΘ, for the following similar reaction, using ΔHf values in section 12 of the data booklet.
3C2H2(g) → C6H6(l)
-
18M.2.sl.TZ1.3b.ii:
Determine the standard enthalpy change, ΔHΘ, for the following similar reaction, using ΔHf values in section 12 of the data booklet.
3C2H2(g) → C6H6(l)
-
21M.2.sl.TZ2.1b:
Thermodynamic data for the decomposition of calcium carbonate is given.
Calculate the enthalpy change of reaction, ΔH, in kJ, for the decomposition of calcium carbonate.
-
18M.2.hl.TZ2.5b.ii:
Determine the value of ΔHΘ, in kJ, for the reaction using the values in the table.
-
21M.2.hl.TZ2.1b(i):
Calculate the enthalpy change of reaction, ΔH, in kJ, for the decomposition of calcium carbonate.
-
20N.1.sl.TZ0.14:
Which combination will give you the enthalpy change for the hydrogenation of ethene to ethane, ?
A.
B.
C.
D.
-
19N.2.hl.TZ0.6a(ii):
Copper(II) chloride is used as a catalyst in the production of chlorine from hydrogen chloride.
4HCl (g) + O2 (g) → 2Cl2 (g) + 2H2O (g)
Calculate the standard enthalpy change, ΔHθ, in kJ, for this reaction, using section 12 of the data booklet.
-
19M.1.sl.TZ2.13:
Consider the following equations.
2Al (s) + O2 (g) → Al2O3 (s) ΔHƟ = −1670 kJ
Mn (s) + O2 (g) → MnO2 (s) ΔHƟ = −520 kJWhat is the standard enthalpy change, in kJ, of the reaction below?
4Al (s) + 3MnO2 (s) → 2Al2O3 (s) + 3Mn (s)
A. −1670 + 520
B. (−1670) + 3(520)
C. 2(−1670) + 3(−520)
D. 2(−1670) + 3(520)
-
17M.2.sl.TZ2.8a.i:
Calculate the enthalpy change, in kJ, for the spray reaction, using the data below.
-
21N.2.sl.TZ0.3c(i):
Calculate the standard enthalpy change (ΔH⦵) for the forward reaction in kJ mol−1.
ΔH⦵f PCl3 (g) = −306.4 kJ mol−1
ΔH⦵f PCl5 (g) = −398.9 kJ mol−1
-
21N.1.sl.TZ0.15:
Which equation represents the standard enthalpy of formation of lithium oxide?
A. 4Li (s) + O2 (g) → 2Li2O (s)B. 2Li (s) + O2 (g) → Li2O (s)
C. Li (s) + O2 (g) → Li2O (s)
D. Li (g) + O2 (g) → Li2O (g)
-
21N.2.hl.TZ0.3c(i):
Calculate the standard enthalpy change (ΔH⦵) for the forward reaction in kJ mol−1.
ΔH⦵f PCl3 (g) = −306.4 kJ mol−1
ΔH⦵f PCl5 (g) = −398.9 kJ mol−1

