| Date | May 2019 | Marks available | 3 | Reference code | 19M.2.hl.TZ1.3 |
| Level | HL | Paper | 2 | Time zone | TZ1 |
| Command term | Determine | Question number | 3 | Adapted from | N/A |
Question
This question is about sodium and its compounds.
The Born-Haber cycle for sodium oxide is shown (not to scale).
Sodium peroxide is used in diving apparatus to produce oxygen from carbon dioxide.
2Na2O2 (s) + 2CO2 (g) → 2Na2CO3 (s) + O2 (g)
Plot the relative values of the first four ionization energies of sodium.
Outline why the alkali metals (group 1) have similar chemical properties.
Describe the structure and bonding in solid sodium oxide.
Calculate values for the following changes using section 8 of the data booklet.
ΔHatomisation (Na) = 107 kJ mol−1
ΔHatomisation (O) = 249 kJ mol−1
O2(g) → O2- (g):
Na (s) → Na+ (g):
The standard enthalpy of formation of sodium oxide is −414 kJ mol−1. Determine the lattice enthalpy of sodium oxide, in kJ mol−1, using section 8 of the data booklet and your answers to (d)(i).
(If you did not get answers to (d)(i), use +850 kJ mol−1 and +600 kJ mol−1 respectively, but these are not the correct answers.)
Justify why K2O has a lower lattice enthalpy (absolute value) than Na2O.
Write equations for the separate reactions of solid sodium oxide and solid phosphorus(V) oxide with excess water and differentiate between the solutions formed.
Sodium oxide, Na2O:
Phosphorus(V) oxide, P4O10:
Differentiation:
Sodium peroxide, Na2O2, is formed by the reaction of sodium oxide with oxygen.
2Na2O (s) + O2 (g) → 2Na2O2 (s)
Calculate the percentage yield of sodium peroxide if 5.00g of sodium oxide produces 5.50g of sodium peroxide.
Determine the enthalpy change, ΔH, in kJ, for this reaction using data from the table and section 12 of the data booklet.
Outline why bond enthalpy values are not valid in calculations such as that in (g)(i).
An allotrope of molecular oxygen is ozone. Compare, giving a reason, the bond enthalpies of the O to O bonds in O2 and O3.
Outline why a real gas differs from ideal behaviour at low temperature and high pressure.
The reaction of sodium peroxide with excess water produces hydrogen peroxide and one other sodium compound. Suggest the formula of this compound.
State the oxidation number of carbon in sodium carbonate, Na2CO3.
Markscheme
[✔]
Notes: Accept curve showing general trend.
Award mark only if the energy difference between the first two points is larger than that between points 2/3 and 3/4.
same number of electrons in outer shell
OR
all are s1 [✔]
«3-D/giant» regularly repeating arrangement «of ions»
OR
lattice «of ions» [✔]
electrostatic attraction between oppositely charged ions
OR
electrostatic attraction between Na+ and O2− ions [✔]
Note: Do not accept “ionic” without description.
O2(g) → O2- (g)
«ΔHatomisation (O) + 1st EA + 2nd EA = 249 k Jmol−1 − 141 kJmol−1 + 753 kJmol−1 =» «+»861 «kJmol−1» [✔]
Na (s) → Na+ (g)
«ΔHatomisation (Na) + 1st IE = 107 kJmol−1 + 496 kJmol−1 =» «+»603 «kJmol−1» [✔]
lattice enthalpy = 861 «kJ mol−1» + 2 × 603 «kJ mol−1» −(−414 «kJ mol−1») [✔]
«= +» 2481 «kJ mol−1» [✔]
Note: Award [2] for correct final answer.
If given values are used:
M1: lattice enthalpy = 850 «kJ mol−1» +
2 × 600 «kJ mol−1» −(−414 «kJ mol−1»)
M2: «= +» 2464 «kJ mol−1»
K+ ion is larger than Na+
OR
smaller attractive force because of greater distance between ion «centres» [✔]
Sodium oxide:
Na2O(s) + H2O(l) → 2NaOH (aq) [✔]
Phosphorus(V) oxide:
P4O10 (s) + 6H2O(l) → 4H3PO4 (aq) [✔]
Differentiation:
NaOH/product of Na2O is alkaline/basic/pH > 7 AND H3PO4/product of P4O10 is acidic/pH < 7 [✔]
n(Na2O2) theoretical yield «= » = 0.0807/8.07 × 10−2 «mol»
OR
mass of Na2O2 theoretical yield «= × 77.98 gmol−1» = 6.291 «g» [✔]
% yield «= × 100» OR « × 100» = 87.4 «%» [✔]
Note: Award [2] for correct final answer.
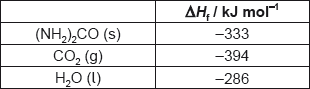
∑ΔHf products = 2 × (−1130.7) / −2261.4 «kJ» [✔]
∑ΔHf reactants = 2 × (−510.9) + 2 × (−393.5) / −1808.8 «kJ» [✔]
ΔH = «∑ΔHf products − ∑ΔHf reactants = −2261.4 −(−1808.8) =» −452.6 «kJ» [✔]
Note: Award [3] for correct final answer.
Award [2 max] for “+ 452.6 «kJ»”.
only valid for covalent bonds
OR
only valid in gaseous state [✔]
bond in O3 has lower enthalpy AND bond order is 1.5 «not 2» [✔]
Note: Accept “bond in ozone is longer”.
Any one of:
finite volume of particles «requires adjustment to volume of gas» [✔]
short-range attractive forces «overcomes low kinetic energy» [✔]
NaOH [✔]
IV [✔]
Examiners report
Generally well done with a correct plot of ionization energies.
The majority answered correctly stating same number of valence electrons as the reason. Some candidates stated same size or similar ionization energy but the majority scored well.
Many candidates lost one or two marks for missing “electrostatic forces” between “oppositely charged ions”, or “lattice”. Some candidates’ answers referred to covalent bonds and shapes of molecules.
Good performance with typical error being in the calculation for the first equation, ½O2 (g) → O2− (g), where the value for the first electron affinity of oxygen was left out.
Many candidates earned some credit for ECF based on (d)(i).
Average performance with answers using atomic size rather than ionic size or making reference to electronegativities of K and Na.
An average of 1.1 out of 3 earned here. Many candidates could write a balanced equation for the reaction of sodium oxide with water but not phosphorus(V) oxide. Mediocre performance in identifying the acid/base nature of the solutions formed.
The majority earned one or two marks in finding a % yield.
The average was 2.2 out 3 for this question on enthalpy of formation. Enthalpy calculations were generally well done.
The majority of candidates referred to “bond enthalpy values are average”, rather than not valid for solids or only used for gases.
Some candidates recognized that ozone had a resonance structure but then only compared bond length between ozone and oxygen rather than bond enthalpy.
Few candidates could distinguish the cause for difference in behaviour between real and ideal gases at low temperature or high pressure. Many answers were based on increase in number of collisions or faster rate or movement of gas particles.
Na2O was a common formula in many candidates’ answers for the product of the reaction of sodium peroxide with water.
The vast majority of candidates could correctly state the oxidation number of carbon in sodium carbonate.