| Date | May 2019 | Marks available | 3 | Reference code | 19M.2.hl.TZ1.4 |
| Level | HL | Paper | 2 | Time zone | TZ1 |
| Command term | Draw | Question number | 4 | Adapted from | N/A |
Question
This question is about the decomposition of hydrogen peroxide.
Hydrogen peroxide decomposes to water and oxygen when a catalyst such as potassium iodide, KI, is added.
2H2O2 (aq) O2 (g) + 2H2O (l)
Suggest why many chemicals, including hydrogen peroxide, are kept in brown bottles instead of clear colourless bottles.
In a laboratory experiment solutions of potassium iodide and hydrogen peroxide were mixed and the volume of oxygen generated was recorded. The volume was adjusted to 0 at t = 0.
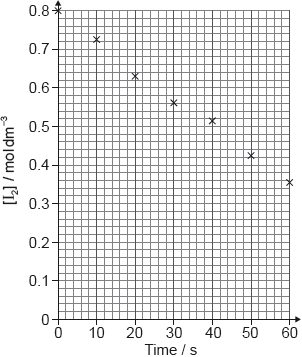
The data for the first trial is given below.
Plot a graph on the axes below and from it determine the average rate of
formation of oxygen gas in cm3 O2 (g) s−1.
Average rate of reaction:
Two more trials (2 and 3) were carried out. The results are given below.
Determine the rate equation for the reaction and its overall order, using your answer from (b)(i).
Rate equation:
Overall order:
Additional experiments were carried out at an elevated temperature. On the axes below, sketch Maxwell–Boltzmann energy distribution curves at two temperatures T1 and T2, where T2 > T1.
Apart from a greater frequency of collisions, explain, by annotating your graphs in (b)(iii), why an increased temperature causes the rate of reaction to increase.
MnO2 is another possible catalyst for the reaction. State the IUPAC name for MnO2.
Comment on why peracetic acid, CH3COOOH, is always sold in solution with ethanoic acid and hydrogen peroxide.
H2O2 (aq) + CH3COOH (aq) ⇌ CH3COOOH (aq) + H2O (l)
Sodium percarbonate, 2Na2CO3•3H2O2, is an adduct of sodium carbonate and hydrogen peroxide and is used as a cleaning agent.
Mr (2Na2CO3•3H2O2) = 314.04
Calculate the percentage by mass of hydrogen peroxide in sodium percarbonate, giving your answer to two decimal places.
Markscheme
decomposes in light [✔]
Note: Accept “sensitive to light”.
points correctly plotted [✔]
best fit line AND extended through (to) the origin [✔]
Average rate of reaction:
«slope (gradient) of line =» 0.022 «cm3 O2 (g) s−1» [✔]
Note: Accept range 0.020–0.024cm3 O2 (g) s−1.
Rate equation:
Rate = k[H2O2] × [KI] [✔]
Overall order:
2 [✔]
Note: Rate constant must be included.
peak of T2 to right of AND lower than T1 [✔]
lines begin at origin AND T2 must finish above T1 [✔]
Ea marked on graph [✔]
explanation in terms of more “particles” with E ≥ Ea
OR
greater area under curve to the right of Ea in T2 [✔]
manganese(IV) oxide
OR
manganese dioxide [✔]
Note: Accept “manganese(IV) dioxide”.
moves «position of» equilibrium to right/products [✔]
Note: Accept “reactants are always present as the reaction is in equilibrium”.
M( H2O2) «= 2 × 1.01 + 2 × 16.00» = 34.02 «g» [✔]
«% H2O2 = 3 × × 100 =» 32.50 «%» [✔]
Note: Award [2] for correct final answer.
Examiners report
There were a couple of comments claiming that this NOS question on “why to store hydrogen peroxide in brown bottles” is not the syllabus. Most candidates were quite capable of reasoning this out.
Most candidates could plot a best fit line and find the slope to calculate an average rate of reaction.
Good performance but with answers that either typically included only [H2O2] with first or second order equation or even suggesting zero order rate equation.
Fair performance; errors including not starting the two curves at the origin, drawing peak for T2 above T1, T2 finishing below T1 or curves crossing the x-axis.
The majority of candidates earned at least one mark, many both marks. Errors included not annotating the graph with Ea and referring to increase of kinetic energy as reason for higher rate at T2.
A well answered question. Very few candidates had problem with nomenclature.
One teacher suggested that “stored” would have been better than “sold” for this question. There were a lot of irrelevant answers with many believing the back reaction was an acid dissociation.
It is recommended that candidates use the relative atomic masses given in the periodic table.