| Date | November 2018 | Marks available | 3 | Reference code | 18N.3.hl.TZ0.1 |
| Level | HL | Paper | 3 | Time zone | TZ0 |
| Command term | Calculate | Question number | 1 | Adapted from | N/A |
Question
Alloys containing at least 60 % copper reduce the presence of bacteria on their surface.The percentage of copper in brass, an alloy of copper and zinc, can be determined by UV-vis spectrometry.
A sample of brass is dissolved in concentrated nitric acid and then made up to 250.0 cm3 with water before analysis.
Cu (s) + 4HNO3 (aq) → Cu(NO3)2 (aq) + 2NO2 (g) + 2H2O (l)
3Zn (s) + 8HNO3 (aq) → 3Zn(NO3)2 (aq) + 2NO (g) + 4H2O (l)
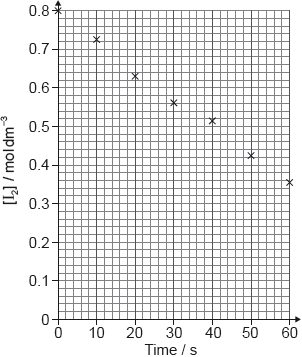
The concentration of copper(II) ions in the resulting solution is then determined from a calibration curve, which is plotted by measuring the light absorbance of standard solutions.
You may find the following chart and diagram helpful.
Outline why the initial reaction should be carried out under a fume hood.
Deduce the equation for the relationship between absorbance and concentration.
Copper(II) ion solutions are blue. Suggest, giving your reason, a suitable wavelength of light for the analysis.
Outline how a solution of 0.0100 mol dm−3 is obtained from a standard 1.000 mol dm−3 copper(II) sulfate solution, including two essential pieces of glassware you would need.
The original piece of brass weighed 0.200 g. The absorbance was 0.32.
Calculate, showing your working, the percentage of copper by mass in the brass.
Deduce the appropriate number of significant figures for your answer in (e)(i).
Comment on the suitability of using brass of this composition for door handles in hospitals.
If you did not obtain an answer to (e)(i), use 70 % but this is not the correct answer.
Suggest another property of brass that makes it suitable for door handles.
Titration is another method for analysing the solution obtained from adding brass to nitric acid.
Copper(II) ions are reduced to copper(I) iodide by the addition of potassium iodide solution, releasing iodine that can be titrated with sodium thiosulfate solution, Na2S2O3 (aq). Copper(I) iodide is a white solid.
4I− (aq) + 2Cu2+ (aq) → 2CuI (s) + I2 (aq)
I2 (aq) + 2S2O32− (aq) → 2I− (aq) + S4O62− (aq)
Suggest why the end point of the titration is difficult to determine, even with the addition of starch to turn the remaining free iodine black.
Markscheme
NO2/NO/NOx/HNO3/gas is poisonous/toxic/irritant ✔
Accept formula or name.
Accept “HNO3 is corrosive” OR “poisonous/toxic gases produced”.
Accept “reaction is harmful/hazardous”.
Slope (gradient):
40 ✔
Equation:
absorbance = 40 × concentration
OR
y = 40x ✔
Accept any correct relationship for slope such as .
Award [2] if equation in M2 is correct.
orange is opposite blue «in the colour wheel»
OR
the complementary colour «blue» is seen/transmitted ✔
585–647 «nm would be absorbed» ✔
Accept any value or range within 550–680 «nm» for M2.
dilute 1.00 cm3 «of the standard solution with water» to 100 cm3
OR
dilute sample of standard solution «with water» 100 times ✔
«graduated/volumetric» pipette/pipet ✔
volumetric flask ✔
Accept any 1 : 100 ratio for M1.
Accept “mix 1 cm3 of the standard solution with 99 cm3 of water” for M1.
Do not accept “add 100 cm3 of water to 1.00 cm3 of standard solution” for M1.
Accept “burette/buret” for M2.
Accept “graduated/measuring flask” for M3 but not “graduated/measuring cylinder” or “conical/Erlenmeyer flask”.
concentration of copper = 0.0080 «mol dm–3» ✔
mass of copper in 250.0 cm3 = «0.0080 mol dm–3 × 0.2500 dm3 × 63.55 g mol–1 =» 0.127 «g»
OR
mass of brass in 1 dm3 = «4 × 0.200 g =» 0.800 g AND [Cu2+] = «0.0080 mol dm–3 × 63.55 g mol–1 =» 0.5084 g dm–3 ✔
«% copper in this sample of brass » 64 «%»
OR
«% copper in this sample of brass » 64 «%» ✔
Accept any value in range 0.0075–0.0085 «mol dm–3» for M1.
Accept annotation on graph for M1.
Award [3] for correct final answer.
Accept “65 «%»”.
two ✔
Do not apply ECF from 1(e)(i).
«since it is greater than 60%» it will reduce the presence of bacteria «on door handles» ✔
resistant to corrosion/oxidation/rusting
OR
low friction surface «so ideal for connected moving components» ✔
Accept “hard/durable”, “«high tensile» strength”, “unreactive”, “malleable” or any reference to the appearance/colour of brass (eg “gold-like”, “looks nice” etc.).
Do not accept irrelevant properties, such as “high melting/boiling point”, “non-magnetic”, “good heat/electrical conductor”, “low volatility”, etc.
Do not accept “ductile”.
precipitate/copper(I) iodide/CuI makes colour change difficult to see
OR
release of I2/iodine from starch-I2 complex is slow so titration must be done slowly ✔