| Date | May 2014 | Marks available | 3 | Reference code | 14M.3.SL.TZ2.5 |
| Level | Standard level | Paper | Paper 3 | Time zone | Time zone 2 |
| Command term | Explain | Question number | 5 | Adapted from | N/A |
Question
This question is about atomic spectra.
The diagram shows some of the energy levels of a hydrogen atom.
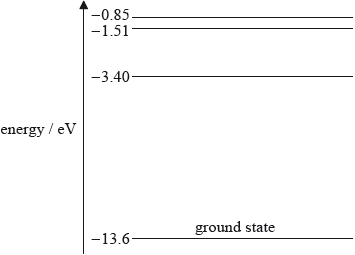
Explain how atomic line spectra provide evidence for the existence of discrete electron energy levels in atoms.
(i) Calculate the wavelength of the photon that will be emitted when an electron moves from the –3.40 eV energy level to the –13.6 eV energy level.
(ii) State and explain if it is possible for a hydrogen atom in the ground state to absorb a photon with an energy of 12.5 eV.
Markscheme
each line represents a single frequency/wavelength;
which corresponds to a specific photon energy / \(E = hf\);
energy of photon determined by energy change of electrons;
electrons transition between energy levels (so discrete energy levels);
Award [3 max] for reverse argument that discrete energy levels produce line spectra.
(i) \(\Delta E = [13.6 - 3.40] \times 1.60 \times {10^{ - 19}}{\text{ (}} = 1.63 \times {10^{ - 18}}{\text{ J)}}\);
\(E = \frac{{hc}}{\lambda }\); (accept implicit use of this equation)
\(\lambda = \frac{{6.63 \times {{10}^{ - 34}} \times 3.00 \times {{10}^8}}}{{1.63 \times {{10}^{ - 18}}}} = 1.22 \times {10^{ - 7}}{\text{ (m)}}\);
Award [3] for a correct bald answer.
(ii) photon absorbed when its energy is equal to the difference between two energy levels;
so absorption not possible;
Examiners report
In (a) the logical sequence is: line spectra ➡ discrete photon energy ➡ discrete electron transitions ➡ discrete electron energy levels. However, very few were able to sequence their answers in this way. Despite this there were many reasonable answers.
In (i) many correct answers were seen, but there were some answers where the de Broglie formula was mistakenly used. (ii) was poorly answered as few could explain that a 12.5eV photon did not match any of the possible transition energies.

