| Date | November 2014 | Marks available | 1 | Reference code | 14N.3.SL.TZ0.6 |
| Level | Standard level | Paper | Paper 3 | Time zone | Time zone 0 |
| Command term | Draw | Question number | 6 | Adapted from | N/A |
Question
This question is about atomic spectra and energy states.
Outline how atomic absorption spectra provide evidence for the quantization of energy states in atoms.
The diagram shows some atomic energy levels of hydrogen.
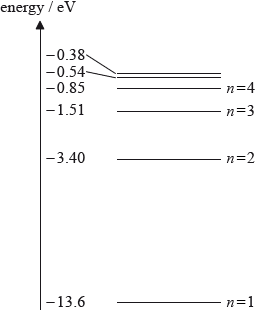
A photon of energy 2.86 eV is emitted from a hydrogen atom. Using the diagram, draw an arrow to indicate the electron transitions that results in the emission of this photon.
Markscheme
an atom will only absorb a photon if the photon energy corresponds to an energy difference between two of its energy states;
the absorption of energy takes places in discrete quantities (quanta);
arrow drawn downwards from \( - 0.54\) level to \( - 3.40\) level;
Examiners report
In (a), candidates rarely described absorption spectra, rather explaining emission spectra. There were very poor explanations of quantized energy states.
(b) was generally well answered, although a common mistake was to have the change in the wrong direction.
Syllabus sections
- 17N.2.HL.TZ0.3a.i: State and explain the nature of the particle labelled X.
- 17N.1.SL.TZ0.23: Which statement about atomic spectra is not true? A. They provide evidence for discrete...
- 17M.2.HL.TZ2.5c.i: The wall of cylinder A is made from glass. Outline why this glass wall had to be very thin.
- 17M.2.HL.TZ2.5a: Write down the nuclear equation for this decay.
- 17M.2.SL.TZ2.4d: Rutherford and Royds identified the helium gas in cylinder B by observing its emission...
- 17M.2.SL.TZ2.4b: Rutherford and Royds put some pure radium-226 in a small closed cylinder A. Cylinder A is...
- 17M.2.SL.TZ2.4a: Write down the missing values in the nuclear equation for this decay.
- 17M.1.HL.TZ2.25: Which of the following leads to a paradigm shift? A. Multi-loop circuits B. Standing...
- 17M.1.HL.TZ1.20: A pure sample of nuclide A and a pure sample of nuclide B have the same activity at time t =...
- 17M.1.SL.TZ2.25: The half-life of a radioactive element is 5.0 days. A freshly-prepared sample contains 128 g...
- 17M.1.SL.TZ2.24: Atomic spectra are caused when a certain particle makes transitions between energy...
- 17M.1.SL.TZ1.24: A nucleus of phosphorus (P) decays to a nucleus of silicon (Si) with the emission of particle...
- 17M.1.SL.TZ1.15: Two pulses are travelling towards each other. What is a possible pulse shape when the...
- 16N.3.SL.TZ0.3b: The change in foam height can be modelled using ideas from other areas of physics. Identify...
- 16N.3.SL.TZ0.3a: Determine the time taken for the foam to drop to (i) half its initial height. (ii) a...
- 16M.2.SL.TZ0.6b: The graph shows the variation with time t of the activity A of a sample containing...
- 16N.1.HL.TZ0.40: What is the charge on an electron antineutrino and during what process is an electron...
- 16N.1.HL.TZ0.20: Which of the following lists the particles emitted during radioactive decay in order of...
- 16N.1.SL.TZ0.24: Photons of energy 2.3eV are incident on a low-pressure vapour. The energy levels of the atoms...
- 16M.1.SL.TZ0.24: ...
- 15M.1.SL.TZ1.23: Nucleus P decays by a sequence of emissions to form nucleus Q. One \(\alpha \) particle and...
- 15M.1.HL.TZ1.31: Some of the energy levels for a hydrogen atom are shown in the diagram. The table shows four...
- 15M.1.SL.TZ2.22: What is the relationship between nucleon number A, proton number Z and neutron number...
- 15M.1.SL.TZ2.24: The initial number of atoms in a pure radioactive sample is N. The radioactive half-life of...
- 15M.2.SL.TZ1.6g: Calculate the percentage of a sample of calcium-47 that decays in 27 days.
- 15M.2.HL.TZ1.9f: For the final thallium nuclide, identify the (i) nucleon number. (ii) proton number.
- 15M.2.HL.TZ1.9g: Radon-220 is a radioactive gas. It is released by rocks such as granite. In some parts of the...
- 15M.2.SL.TZ2.4e: U-235 \(\left( {{}_{92}^{235}{\rm{U}}} \right)\) can undergo alpha decay to form an isotope...
- 15M.3.SL.TZ2.6a: An electron is excited to the n=3 energy level. On the diagram, draw arrows to show the...
- 15M.3.SL.TZ2.6b: Show that a photon of wavelength 656 nm can be emitted from a hydrogen atom.
- 14M.1.SL.TZ1.24: A radioactive sample has activity A0 at t=0. What will be the activity of the sample after...
- 14M.1.HL.TZ1.32: The de Broglie wavelength of an electron is equal to the wavelength of a photon that has...
- 14M.1.HL.TZ1.29: The arrows below indicate transitions involving three energy levels of an atom. The...
- 14M.1.HL.TZ1.30: The graph shows the variation with time t of the activity A of a radioactive sample. The...
- 14M.1.SL.TZ2.22: Which of the following provides evidence for the existence of atomic energy levels? A....
- 14M.1.SL.TZ2.24: Nuclei of the isotope nitrogen-14 are bombarded with neutrons and as a result nuclei of an...
- 14M.1.HL.TZ2.30: The diagram shows four energy levels W, X, Y and Z of an atom. Which electron transition...
- 14M.1.HL.TZ2.32: The nuclei in a sample of a radioactive isotope decay by emitting α and γ particles. Which of...
- 14M.1.HL.TZ2.33: A pure sample of a known element has a very long half-life. What measurement(s), together...
- 15N.1.HL.TZ0.31: All the energy levels in a simple model of an atom are shown. The atom is excited so that...
- 15N.1.HL.TZ0.33: \(_{\;{\text{6}}}^{{\text{11}}}{\text{C}}\) undergoes \({\beta ^ + }\) decay. The products of...
- 15N.2.HL.TZ0.6c.ii: Construct the nuclear equation for the decay of radium-226.
- 15N.1.SL.TZ0.24: A simple model of the hydrogen atom suggests that the electron orbits the proton. What is the...
- 15N.2.SL.TZ0.4b: Outline why classical physics does not permit a model of an electron orbiting the nucleus.
- 15N.2.SL.TZ0.4c.i: State what is meant by the terms nuclide and isotope. Nuclide: Isotope:
- 15N.2.SL.TZ0.4c.iii: Radium-226 has a half-life of 1600 years. Determine the time, in years, it takes for the...
- 14N.1.SL.TZ0.24: In a neutral atom there are ne electrons, np protons and nn neutrons. What is the mass number...
- 15N.3.SL.TZ0.7a: Aluminium-26 decays into an isotope of magnesium (Mg) by \({\beta ^ + }\)...
- 15N.3.SL.TZ0.7b: Explain why the beta particles emitted from the aluminium-26 have a continuous range of...
- 14N.1.HL.TZ0.34: A radioactive nuclide decays to a stable daughter nuclide. Initially the sample consists...
- 14N.2.SL.TZ0.3a: Explain what is meant by an isotope.
- 14N.2.SL.TZ0.3c.ii: A different isotope has half the initial activity and double the half-life of I-131. On the...
- 14N.2.SL.TZ0.3b: Identify the missing entries to complete the nuclear reaction for the decay of I-131.
- 14N.2.SL.TZ0.3c.i: The I-131 can be used for a medical application but only when the activity lies within the...
- 14N.3.SL.TZ0.6a: Outline how atomic absorption spectra provide evidence for the quantization of energy states...
- 14N.3.SL.TZ0.7a.i: Identify the numbers and the particle to complete the decay equation.
- 14N.3.SL.TZ0.7a.ii: State the nature of the \({\beta ^ + }\) particle.
- 14M.3.SL.TZ2.5a: Explain how atomic line spectra provide evidence for the existence of discrete electron...
- 14M.3.SL.TZ2.5b: (i) Calculate the wavelength of the photon that will be emitted when an electron moves...
- 11N.1.SL.TZO.22: A nucleus of the isotope plutonium-238 \(\left( {{}^{238}{\rm{P}}} \right)\) decays into a...
- 11N.1.SL.TZO.24: Which of the following affects the rate at which a sample of a radioactive material...
- 11N.1.HL.TZ0.31: A proton decays to a neutron. The other products of the decay are a A. positron and...
- 11N.1.HL.TZ0.32: The half-life of a radioactive nuclide is 20s. What fraction of the original sample will have...
- 11N.1.HL.TZ0.33: Which of the following gives evidence to support the existence of atomic energy levels? A....
- 12N.1.HL.TZ0.23: The diagram shows three electron energy levels of an atom. Which transition results in the...
- 13N.1.SL.TZ0.23: In a particular atom, the nucleon number is the total number of A. protons.B. neutrons.C....
- 13N.1.HL.TZ0.29: The diagram shows the three lowest energy levels of an atom. Which diagram shows the...
- 13M.1.HL.TZ1.27: Which of the following would decrease the initial activity of a sample of plutonium? A....
- 12M.1.SL.TZ2.25: The half-life of a particular radioactive isotope is 8 days. The initial activity of a pure...
- 12M.1.SL.TZ2.22: The nuclear reaction equation for the decay of a nucleus of thorium-231 (Th-231) to a nucleus...
- 12M.1.SL.TZ1.22: When compared with beta particles and gamma-ray photons, alpha particles have the...
- 12M.1.HL.TZ2.30: The diagram shows three energy levels of the hydrogen atom and some of the associated...
- 13M.2.SL.TZ2.4e: A sample of tritium has an activity of 8.0×104 Bq at time t=0. The half-life of tritium is 12...
- 13M.2.SL.TZ2.4a: The isotope tritium (hydrogen-3) has a radioactive half-life of 12 days. (i) State what is...
- 13M.3.SL.TZ1.5a: Explain how atomic spectra provide evidence for the quantization of energy in atoms.
- 13M.3.SL.TZ1.6a: Identify the missing entries in the following nuclear...
- 13M.3.SL.TZ1.6b: Define half-life.
- 12M.2.SL.TZ2.3a: The nuclide U-235 is an isotope of uranium. A nucleus of U-235 undergoes radioactive decay to...
- 11M.1.HL.TZ2.29: ...
- 12M.1.HL.TZ1.27: When compared with beta particles and gamma-ray photons, alpha particles have the...
- 12M.1.HL.TZ1.25: All isotopes of uranium must have the same A. chemical properties.B. mass.C. half-life.D....
- 12M.1.HL.TZ1.30: The lowest four energy levels of a particular atom are represented in the energy level...
- 12M.1.HL.TZ1.32: Evidence for the existence of isotopes can come from analysis of A. the closest approach...
- 13M.1.SL.TZ2.23: A nucleus of californium (Cf) contains 98 protons and 154 neutrons. Which of the following...
- 11M.2.SL.TZ2.5e: A nucleus of the isotope O-19...
- 11M.2.SL.TZ2.5d: The reaction in (c) produces oxygen...
- 12M.2.SL.TZ1.5a: Describe the phenomenon of natural radioactive decay.
- 12M.2.SL.TZ1.5b: A nucleus of americium-241 (Am-241) decays into a nucleus of neptunium-237 (Np-237) in the...
- 12M.2.HL.TZ1.15b: Outline how atomic emission spectra provide evidence for the quantization of energy in atoms.
- 12M.2.HL.TZ2.14a: The diagram represents the three principal spectral lines in the visible region of...
- 11N.2.HL.TZ0.13a: (i) Calculate, in eV, the energy of a photon of wavelength 490 nm. (ii) On the diagram...
- 11N.2.HL.TZ0.3a: Describe what is meant by (i) radioactive decay. (ii) nuclear fusion.
- 12N.3.SL.TZ0.6c: State the quantities that need to be measured in order to determine the half-life of a...
- 12N.2.SL.TZ0.3c: Explain, with reference to the biological effects of ionizing radiation, why it is important...
- 12N.2.SL.TZ0.3a: State the nuclear equation for this reaction.
- 12N.3.SL.TZ0.5b: (i) Describe the appearance of an atomic absorption spectrum. (ii) Explain why the spectrum...
- 12N.3.SL.TZ0.5a: Outline a laboratory procedure for producing and observing the atomic absorption spectrum of...
- 12N.3.SL.TZ0.5c: The principal energy levels of the hydrogen atom in electronvolt (eV) are given...
- 13N.2.SL.TZ0.4f: A nucleus of another isotope of the element X in (d) decays with a...
- 13N.2.HL.TZ0.10d: The diagram shows four spectral lines in the visible line emission spectrum of atomic...
- 13N.2.HL.TZ0.10e: The energies of the principal energy levels in atomic hydrogen measured in eV are given by...
- 11M.1.SL.TZ1.22: Which of the following gives the correct number of protons and neutrons in a nucleus...
- 11M.1.SL.TZ1.23: A freshly prepared sample contains 4.0 μg of iodine-131. After 24 days, 0.5μg of iodine-131...
- 11M.2.SL.TZ1.5a: For particle P, (i) state how graph 1 shows that its oscillations are not damped. (ii)...
- 11M.2.SL.TZ1.4d: During its normal operation, the following set of reactions takes place in the...
- 11M.3.SL.TZ1.5a: Deduce that the energy of a photon of wavelength 658 nm is 1.89 eV.
- 11M.3.SL.TZ1.5b: (i) On diagram 1, draw an arrow to show the electron transition between energy levels that...
- 11M.3.SL.TZ1.5c: Explain why the lines in the emission spectrum of atomic hydrogen, shown in diagram 2, become...
- 11M.3.SL.TZ1.6a: State the reaction for the decay of the I-124 nuclide.
- 11M.3.SL.TZ1.6b: The graph below shows how the activity of a sample of iodine-124 changes with time. (i)...
- 09M.1.HL.TZ1.28: The diagram shows four possible electron energy levels in the hydrogen atom. The number of...
- 09M.1.HL.TZ1.33: When a nucleus undergoes radioactive \({\beta ^ + }\) decay, the change in the number of...
- 09M.1.SL.TZ1.22: The number of neutrons and the number of protons in a nucleus of an atom of the isotope of...
- 10M.1.HL.TZ1.32: A nucleus of the isotope potassium-40 decays to a nucleus of the isotope argon-40. The...
- 09N.1.SL.TZ0.22: The relationship between proton number \(Z\), neutron number \(N\) and nucleon number \(A\)...
- 10M.1.SL.TZ1.24: Which of the following correctly identifies the three particles emitted in the decay of the...
- 09N.1.SL.TZ0.24: A radio-isotope has an activity of 400 Bq and a half-life of 8 days. After 32 days the...
- 10N.1.SL.TZ0.24: A radioactive isotope has a half-life of two minutes. A sample contains sixteen grams of the...
- 09N.1.HL.TZ0.30: A radioactive isotope has an initial activity \({A_0}\) and a half-life of 1 day. The graph...
- 10N.1.HL.TZ0.33: The energies of alpha particles and of gamma-rays emitted in radioactive decay are discrete....
- 10M.1.SL.TZ1.22: Emission and absorption spectra provide evidence for A. the nuclear model of the...
- 10N.1.SL.TZ0.26: Which of the following is true about beta minus (\({\beta ^ - }\)) decay? A. An...
- 10N.3.SL.TZ0.B2b: Calculate the difference in energy in eV between the energy levels in the hydrogen atom that...
- 10N.3.SL.TZ0.B3a: A nucleus of a radioactive isotope of gold (Au-189) emits a neutrino in the decay to a...

