| Date | May 2009 | Marks available | 3 | Reference code | 09M.2.sl.TZ2.3 |
| Level | SL | Paper | 2 | Time zone | TZ2 |
| Command term | Determine | Question number | 3 | Adapted from | N/A |
Question
Smog is common in cities throughout the world. One component of smog is PAN (peroxyacylnitrate) which consists of 20.2% C, 11.4% N, 65.9% O and 2.50% H by mass. Determine the empirical formula of PAN, showing your working.
Markscheme
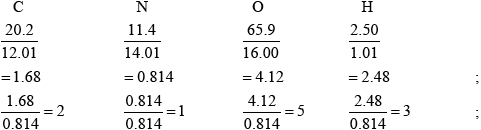
\({{\text{C}}_{\text{2}}}{\text{N}}{{\text{O}}_{\text{5}}}{{\text{H}}_{\text{3}}}\);
No penalty for use of 12, 1 and/or 14.
Award [1 max] if the empirical formula is correct, but no working shown.
Examiners report
It was pleasing to see the majority of candidates determine the correct empirical formula of PAN. Also, candidates showed the proper working with all the appropriate steps.
Syllabus sections
Show 77 related questions
- 17N.3.sl.TZ0.8a.i: Determine the empirical formula of linoleic acid.
- 17N.3.sl.TZ0.3c: Calculate the percentage of water by mass in the NaCl•2H2O crystals. Use the data from...
- 17N.2.sl.TZ0.4a: Complete combustion of 0.1595 g of menthol produces 0.4490 g of carbon dioxide and 0.1840 g...
- 17N.2.sl.TZ0.1d.ii: Calculate the enthalpy change, ΔH, in kJ mol–1, for the reaction between ethanoic acid and...
- 17N.1.sl.TZ0.3: How many grams of sodium azide, NaN3, are needed to produce 68.1 dm3 of N2 (g) at STP? Molar...
- 17N.1.sl.TZ0.2: What is the value of x when 32.2 g of Na2SO4•xH2O are heated leaving 14.2 g of...
- 17N.1.sl.TZ0.1: How many atoms of nitrogen are there in 0.50 mol of (NH4)2CO3? A. 1 B. 2 C. 3.01 ×...
- 17N.2.hl.TZ0.2d.iii: Determine the molecular formula of menthol using your answers from parts (d)(i) and (ii).
- 17N.2.hl.TZ0.2d.i: Complete combustion of 0.1595 g of menthol produces 0.4490 g of carbon dioxide and 0.1840 g...
- 17N.1.hl.TZ0.4: A compound with Mr = 102 contains 58.8 % carbon, 9.80 % hydrogen and 31 % oxygen by mass.What...
- 17N.1.hl.TZ0.2: Which solution neutralizes 50.0 cm3 of 0.120 mol dm–3 NaOH (aq)? A. 12.5 cm3 of 0.080 mol...
- 17M.2.sl.TZ2.7b.ii: Calculate the molar mass of the acid.
- 17M.2.sl.TZ2.1a.i: After heating 3.760 g of a silver oxide 3.275 g of silver remained. Determine the empirical...
- 17M.1.sl.TZ2.2: How many moles of oxygen atoms are there in 0.500 mol of hydrated iron(II) ammonium...
- 17M.3.hl.TZ1.15d: The sequence of nitrogenous bases in DNA determines hereditary characteristics. Calculate...
- 17M.2.hl.TZ1.6c.i: One possible product, X, of the reaction of ethane with chlorine has the...
- 17M.2.sl.TZ1.5c.i: One possible product, X, of the reaction of ethane with chlorine has the...
- 17M.1.sl.TZ1.1: Which compound has the greatest percentage by mass of nitrogen atoms? A. N2H4 B. ...
- 16N.3.sl.TZ0.18b: Suggest why isolation of the crude product involved the addition of ice-cold water.
- 16N.3.sl.TZ0.2c: List two assumptions made in this experiment.
- 16N.3.sl.TZ0.2a: State and explain the further work students need to carry out in trial 2 before they can...
- 16M.2.hl.TZ0.1d: Impurities cause phosphine to ignite spontaneously in air to form an oxide of phosphorus and...
- 16M.2.sl.TZ0.2a: (i) 200.0 g of air was heated by the energy from the complete combustion of 1.00 mol...
- 16M.1.sl.TZ0.2: For which compound is the empirical formula the same as the molecular...
- 15M.1.sl.TZ1.2: Which sample contains the largest amount, in mol, of oxygen atoms? A. 0.20 mol...
- 15M.1.sl.TZ1.3: Which compound has the highest percentage of carbon by mass? A. ...
- 15M.1.sl.TZ2.1: What is the total number of protons and electrons in one mole of hydrogen gas? A. 2 B....
- 15M.1.sl.TZ2.2: A hydrocarbon contains 85.7 % carbon by mass. What is the empirical formula of the...
- 15M.2.sl.TZ1.7a: A hydrocarbon has the empirical formula \({{\text{C}}_{\text{3}}}{{\text{H}}_{\text{7}}}\)....
- 14M.2.hl.TZ1.3b: Calculate, showing your working, the relative atomic mass, \({A_{\text{r}}}\), of magnesium,...
- 14M.2.hl.TZ2.1a: The density of ethanoic acid is \({\text{1.05 g}}\,{\text{c}}{{\text{m}}^{ - 3}}\). Determine...
- 14M.3.hl.TZ1.2a: Show that the empirical formula of compound X is...
- 14M.1.sl.TZ1.2: The structural formula of a dioxin is shown below. What is its empirical formula? A. ...
- 14M.2.sl.TZ1.2b: (i) Define the term relative atomic mass. (ii) Calculate, showing your...
- 14M.1.sl.TZ2.3: For which compounds is the empirical formula the same as the molecular formula? I. ...
- 14M.1.sl.TZ2.1: What is the mass, in g, of one mole of hydrated copper(II) sulfate,...
- 14N.2.sl.TZ0.4b: Deduce the empirical formula of D-fructose.
- 14N.2.sl.TZ0.4c: Calculate the percentage composition by mass of D-fructose.
- 13N.1.sl.TZ0.2: Which represents an empirical formula? A. ...
- 13M.2.hl.TZ1.1d: Determine the molecular formula of HA.
- 13M.1.sl.TZ1.2: What is the molar mass, in \({\text{g}}\,{\text{mo}}{{\text{l}}^{ - 1}}\), of a substance if...
- 13M.1.sl.TZ1.1: Which statements are correct about Avogadro’s constant? I. It is the number of ions in...
- 13M.2.sl.TZ1.1b: This known mass of acid, HA, was then dissolved in distilled water to form a...
- 13M.2.sl.TZ1.1c: The percentage composition of HA is 70.56% carbon, 23.50% oxygen and 5.94% hydrogen....
- 13M.2.hl.TZ2.8e.ii: Menthol occurs naturally and has several isomers. State the structural feature of menthol...
- 13M.2.hl.TZ2.8e.i: When a \(6.234 \times {10^{ - 2}}{\text{ g}}\) of the compound was combusted,...
- 13M.1.sl.TZ2.1: Which contains the largest number of ions? A. 1 mol...
- 13M.1.sl.TZ2.3: Which is the best description of relative atomic mass, Ar? A. The number of neutrons and...
- 13M.2.sl.TZ2.7d.i: Determine its empirical formula.
- 13M.2.sl.TZ2.7d.ii: Determine its molecular formula given that its molar mass is...
- 12N.2.hl.TZ0.7b.ii: The molar mass of the ester is \({\text{116.18 g}}\,{\text{mo}}{{\text{l}}^{ - 1}}\)....
- 12N.2.hl.TZ0.7b.i: Determine the empirical formula of the ester, showing your working.
- 12N.1.sl.TZ0.2: What is the molar mass, in \({\text{g}}\,{\text{mo}}{{\text{l}}^{ - 1}}\), of washing soda...
- 12N.1.sl.TZ0.1: What is the number of ions in 0.20 mol of...
- 12N.2.sl.TZ0.6b.ii: Determine the empirical formula of the ester, showing your working.
- 12N.2.sl.TZ0.6b.iii: The molar mass of the ester is \({\text{116.18 g}}\,{\text{mo}}{{\text{l}}^{ - 1}}\)....
- 10N.1.hl.TZ0.2: \({\text{300 c}}{{\text{m}}^{\text{3}}}\) of water is added to a solution of...
- 10N.1.sl.TZ0.1: What is the total number of nitrogen atoms in two mol of...
- 10N.2.sl.TZ0.5a: The alkane contains 81.7% by mass of carbon. Determine its empirical formula, showing your...
- 10N.2.sl.TZ0.5b: Equal volumes of carbon dioxide and the unknown alkane are found to have the same mass,...
- 09N.1.sl.TZ0.1: Which non-metal forms an oxide XO2 with a relative molecular mass of 60? A. C B. ...
- 09N.1.sl.TZ0.3: 4.00 mol of a hydrocarbon with an empirical formula of \({\text{C}}{{\text{H}}_{\text{2}}}\)...
- 10M.1.sl.TZ2.1: What is the coefficient of \({\rm{F}}{{\rm{e}}_3}{{\rm{O}}_4}\) when the following equation...
- 10M.1.sl.TZ2.3: Which molecular formula is also an empirical formula? A. ...
- 10M.1.sl.TZ2.4: Which of the following is consistent with Avogadro’s law? A. \(\frac{P}{T} = \) constant...
- 09M.2.hl.TZ1.7c.ii: Give the formulas of all the ions present in the solution.
- 09M.1.sl.TZ1.1: The molar mass of a compound is approximately...
- 09M.1.sl.TZ1.2: Which compound has the empirical formula with the largest mass? A. ...
- 09M.3.sl.TZ1.E2c.iii: 0.0002 moles of \({{\text{I}}^ - }\) were formed in step 3. Calculate the amount, in moles,...
- 11M.2.hl.TZ1.3a: Explain why the relative atomic mass of cobalt is greater than the relative atomic mass of...
- 11M.1.sl.TZ1.3: How many molecules are present in a drop of ethanol,...
- 11M.1.sl.TZ1.4: Which sample has the greatest mass? A. 1 mol of...
- 11M.1.sl.TZ1.5: The relative molecular mass of a gas is 56 and its empirical formula is...
- 11M.2.sl.TZ1.2a: Explain why the relative atomic mass of argon is greater than the relative atomic mass of...
- 12M.1.sl.TZ2.1: What is the total number of atoms in 0.100 mol of...
- 12M.2.sl.TZ2.5a: (i) Distinguish between the terms empirical formula and molecular formula. Empirical...
- 11N.2.sl.TZ0.7d.iii: The molar mass was determined to be \({\text{131.38 g}}\,{\text{mo}}{{\text{l}}^{ - 1}}\)....

