| Date | May 2014 | Marks available | 2 | Reference code | 14M.2.hl.TZ1.3 |
| Level | HL | Paper | 2 | Time zone | TZ1 |
| Command term | Calculate | Question number | 3 | Adapted from | N/A |
Question
Magnesium has three stable isotopes, \(^{{\text{24}}}{\text{Mg}}\), \(^{{\text{25}}}{\text{Mg}}\) and \(^{{\text{26}}}{\text{Mg}}\). The relative abundance of each isotope is 78.99%, 10.00% and 11.01%, respectively, and can be determined using a mass spectrometer.
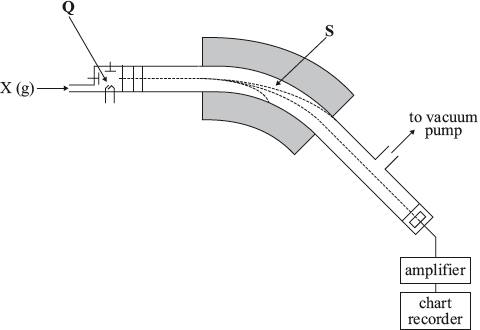
Calculate, showing your working, the relative atomic mass, \({A_{\text{r}}}\), of magnesium, giving your answer to two decimal places.
Markscheme
\(({A_{\text{r}}} = ){\text{ }}0.7899 \times 24 + 0.1000 \times 25 + 0.1101 \times 26\);
24.32;
Award [2] for correct final answer.
Award [1 max] for 24.31 with correct working.
Award [0] for 24.31 (Data Booklet value) if working is incorrect or no working is shown.
Final answer must be to 2 decimal places to score [2].
Examiners report
This question generally scored well. The processes involved in a mass spectrometer were well understood; except for the importance of forming a cation. The calculation of \({A_{\text{r}}}\) and the requirement to give an answer to two decimal places was performed very well.

