| Date | May 2014 | Marks available | 1 | Reference code | 14M.1.sl.TZ2.1 |
| Level | SL | Paper | 1 | Time zone | TZ2 |
| Command term | Question number | 1 | Adapted from | N/A |
Question
What is the mass, in g, of one mole of hydrated copper(II) sulfate, \({\text{CuS}}{{\text{O}}_{\text{4}}} \bullet {\text{5}}{{\text{H}}_{\text{2}}}{\text{O}}\), given the following relative atomic mass values?
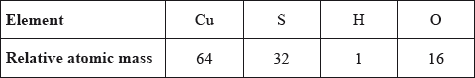
A. 160
B. 178
C. 186
D. 250
Markscheme
D
Examiners report
[N/A]
Syllabus sections
Show 77 related questions
- 17N.3.sl.TZ0.8a.i: Determine the empirical formula of linoleic acid.
- 17N.3.sl.TZ0.3c: Calculate the percentage of water by mass in the NaCl•2H2O crystals. Use the data from...
- 17N.2.sl.TZ0.4a: Complete combustion of 0.1595 g of menthol produces 0.4490 g of carbon dioxide and 0.1840 g...
- 17N.2.sl.TZ0.1d.ii: Calculate the enthalpy change, ΔH, in kJ mol–1, for the reaction between ethanoic acid and...
- 17N.1.sl.TZ0.3: How many grams of sodium azide, NaN3, are needed to produce 68.1 dm3 of N2 (g) at STP? Molar...
- 17N.1.sl.TZ0.2: What is the value of x when 32.2 g of Na2SO4•xH2O are heated leaving 14.2 g of...
- 17N.1.sl.TZ0.1: How many atoms of nitrogen are there in 0.50 mol of (NH4)2CO3? A. 1 B. 2 C. 3.01 ×...
- 17N.2.hl.TZ0.2d.iii: Determine the molecular formula of menthol using your answers from parts (d)(i) and (ii).
- 17N.2.hl.TZ0.2d.i: Complete combustion of 0.1595 g of menthol produces 0.4490 g of carbon dioxide and 0.1840 g...
- 17N.1.hl.TZ0.4: A compound with Mr = 102 contains 58.8 % carbon, 9.80 % hydrogen and 31 % oxygen by mass.What...
- 17N.1.hl.TZ0.2: Which solution neutralizes 50.0 cm3 of 0.120 mol dm–3 NaOH (aq)? A. 12.5 cm3 of 0.080 mol...
- 17M.2.sl.TZ2.7b.ii: Calculate the molar mass of the acid.
- 17M.2.sl.TZ2.1a.i: After heating 3.760 g of a silver oxide 3.275 g of silver remained. Determine the empirical...
- 17M.1.sl.TZ2.2: How many moles of oxygen atoms are there in 0.500 mol of hydrated iron(II) ammonium...
- 17M.3.hl.TZ1.15d: The sequence of nitrogenous bases in DNA determines hereditary characteristics. Calculate...
- 17M.2.hl.TZ1.6c.i: One possible product, X, of the reaction of ethane with chlorine has the...
- 17M.2.sl.TZ1.5c.i: One possible product, X, of the reaction of ethane with chlorine has the...
- 17M.1.sl.TZ1.1: Which compound has the greatest percentage by mass of nitrogen atoms? A. N2H4 B. ...
- 16N.3.sl.TZ0.18b: Suggest why isolation of the crude product involved the addition of ice-cold water.
- 16N.3.sl.TZ0.2c: List two assumptions made in this experiment.
- 16N.3.sl.TZ0.2a: State and explain the further work students need to carry out in trial 2 before they can...
- 16M.2.hl.TZ0.1d: Impurities cause phosphine to ignite spontaneously in air to form an oxide of phosphorus and...
- 16M.2.sl.TZ0.2a: (i) 200.0 g of air was heated by the energy from the complete combustion of 1.00 mol...
- 16M.1.sl.TZ0.2: For which compound is the empirical formula the same as the molecular...
- 15M.1.sl.TZ1.2: Which sample contains the largest amount, in mol, of oxygen atoms? A. 0.20 mol...
- 15M.1.sl.TZ1.3: Which compound has the highest percentage of carbon by mass? A. ...
- 15M.1.sl.TZ2.1: What is the total number of protons and electrons in one mole of hydrogen gas? A. 2 B....
- 15M.1.sl.TZ2.2: A hydrocarbon contains 85.7 % carbon by mass. What is the empirical formula of the...
- 15M.2.sl.TZ1.7a: A hydrocarbon has the empirical formula \({{\text{C}}_{\text{3}}}{{\text{H}}_{\text{7}}}\)....
- 14M.2.hl.TZ1.3b: Calculate, showing your working, the relative atomic mass, \({A_{\text{r}}}\), of magnesium,...
- 14M.2.hl.TZ2.1a: The density of ethanoic acid is \({\text{1.05 g}}\,{\text{c}}{{\text{m}}^{ - 3}}\). Determine...
- 14M.3.hl.TZ1.2a: Show that the empirical formula of compound X is...
- 14M.1.sl.TZ1.2: The structural formula of a dioxin is shown below. What is its empirical formula? A. ...
- 14M.2.sl.TZ1.2b: (i) Define the term relative atomic mass. (ii) Calculate, showing your...
- 14M.1.sl.TZ2.3: For which compounds is the empirical formula the same as the molecular formula? I. ...
- 14N.2.sl.TZ0.4b: Deduce the empirical formula of D-fructose.
- 14N.2.sl.TZ0.4c: Calculate the percentage composition by mass of D-fructose.
- 13N.1.sl.TZ0.2: Which represents an empirical formula? A. ...
- 13M.2.hl.TZ1.1d: Determine the molecular formula of HA.
- 13M.1.sl.TZ1.2: What is the molar mass, in \({\text{g}}\,{\text{mo}}{{\text{l}}^{ - 1}}\), of a substance if...
- 13M.1.sl.TZ1.1: Which statements are correct about Avogadro’s constant? I. It is the number of ions in...
- 13M.2.sl.TZ1.1b: This known mass of acid, HA, was then dissolved in distilled water to form a...
- 13M.2.sl.TZ1.1c: The percentage composition of HA is 70.56% carbon, 23.50% oxygen and 5.94% hydrogen....
- 13M.2.hl.TZ2.8e.ii: Menthol occurs naturally and has several isomers. State the structural feature of menthol...
- 13M.2.hl.TZ2.8e.i: When a \(6.234 \times {10^{ - 2}}{\text{ g}}\) of the compound was combusted,...
- 13M.1.sl.TZ2.1: Which contains the largest number of ions? A. 1 mol...
- 13M.1.sl.TZ2.3: Which is the best description of relative atomic mass, Ar? A. The number of neutrons and...
- 13M.2.sl.TZ2.7d.i: Determine its empirical formula.
- 13M.2.sl.TZ2.7d.ii: Determine its molecular formula given that its molar mass is...
- 12N.2.hl.TZ0.7b.ii: The molar mass of the ester is \({\text{116.18 g}}\,{\text{mo}}{{\text{l}}^{ - 1}}\)....
- 12N.2.hl.TZ0.7b.i: Determine the empirical formula of the ester, showing your working.
- 12N.1.sl.TZ0.2: What is the molar mass, in \({\text{g}}\,{\text{mo}}{{\text{l}}^{ - 1}}\), of washing soda...
- 12N.1.sl.TZ0.1: What is the number of ions in 0.20 mol of...
- 12N.2.sl.TZ0.6b.ii: Determine the empirical formula of the ester, showing your working.
- 12N.2.sl.TZ0.6b.iii: The molar mass of the ester is \({\text{116.18 g}}\,{\text{mo}}{{\text{l}}^{ - 1}}\)....
- 10N.1.hl.TZ0.2: \({\text{300 c}}{{\text{m}}^{\text{3}}}\) of water is added to a solution of...
- 10N.1.sl.TZ0.1: What is the total number of nitrogen atoms in two mol of...
- 10N.2.sl.TZ0.5a: The alkane contains 81.7% by mass of carbon. Determine its empirical formula, showing your...
- 10N.2.sl.TZ0.5b: Equal volumes of carbon dioxide and the unknown alkane are found to have the same mass,...
- 09N.1.sl.TZ0.1: Which non-metal forms an oxide XO2 with a relative molecular mass of 60? A. C B. ...
- 09N.1.sl.TZ0.3: 4.00 mol of a hydrocarbon with an empirical formula of \({\text{C}}{{\text{H}}_{\text{2}}}\)...
- 10M.1.sl.TZ2.1: What is the coefficient of \({\rm{F}}{{\rm{e}}_3}{{\rm{O}}_4}\) when the following equation...
- 10M.1.sl.TZ2.3: Which molecular formula is also an empirical formula? A. ...
- 10M.1.sl.TZ2.4: Which of the following is consistent with Avogadro’s law? A. \(\frac{P}{T} = \) constant...
- 09M.2.hl.TZ1.7c.ii: Give the formulas of all the ions present in the solution.
- 09M.1.sl.TZ1.1: The molar mass of a compound is approximately...
- 09M.1.sl.TZ1.2: Which compound has the empirical formula with the largest mass? A. ...
- 09M.3.sl.TZ1.E2c.iii: 0.0002 moles of \({{\text{I}}^ - }\) were formed in step 3. Calculate the amount, in moles,...
- 09M.2.sl.TZ2.3: Smog is common in cities throughout the world. One component of smog is PAN...
- 11M.2.hl.TZ1.3a: Explain why the relative atomic mass of cobalt is greater than the relative atomic mass of...
- 11M.1.sl.TZ1.3: How many molecules are present in a drop of ethanol,...
- 11M.1.sl.TZ1.4: Which sample has the greatest mass? A. 1 mol of...
- 11M.1.sl.TZ1.5: The relative molecular mass of a gas is 56 and its empirical formula is...
- 11M.2.sl.TZ1.2a: Explain why the relative atomic mass of argon is greater than the relative atomic mass of...
- 12M.1.sl.TZ2.1: What is the total number of atoms in 0.100 mol of...
- 12M.2.sl.TZ2.5a: (i) Distinguish between the terms empirical formula and molecular formula. Empirical...
- 11N.2.sl.TZ0.7d.iii: The molar mass was determined to be \({\text{131.38 g}}\,{\text{mo}}{{\text{l}}^{ - 1}}\)....

