| Date | May 2014 | Marks available | 3 | Reference code | 14M.2.sl.TZ1.2 |
| Level | SL | Paper | 2 | Time zone | TZ1 |
| Command term | Calculate and Define | Question number | 2 | Adapted from | N/A |
Question
Magnesium has three stable isotopes, \(^{{\text{24}}}{\text{Mg}}\), \(^{{\text{25}}}{\text{Mg}}\) and \(^{{\text{26}}}{\text{Mg}}\). The relative abundance of each isotope is 78.99%, 10.00% and 11.01% respectively, and can be determined using a mass spectrometer.
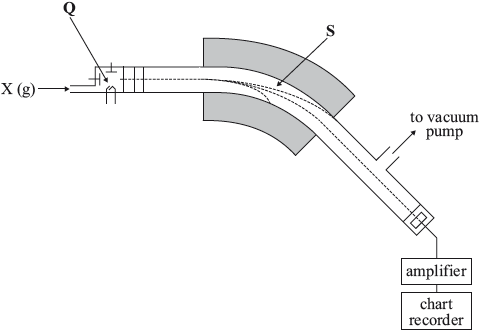
(i) Define the term relative atomic mass.
(ii) Calculate, showing your working, the relative atomic mass, \({A_{\text{r}}}\), of magnesium, giving your answer to two decimal places.
Markscheme
(i) ratio of average/mean mass of atom to \(\frac{{\text{1}}}{{{\text{12}}}}\) of mass of C–12 (isotope) / average/mean mass of atom on scale where one atom of C–12 has mass of 12 / weighted average/mean mass of isotopes of element compared to \(\frac{{\text{1}}}{{{\text{12}}}}\) of mass of C–12 / OWTTE;
Award no mark if “element” is used instead of “atom” in first two alternatives.
Allow “mass of an atom relative to the mass of \(\frac{1}{{12}}\) of C–12”.
(ii) \(({A_{\text{r}}} = ){\text{ }}0.7899 \times 24 + 0.1000 \times 25 + 0.1101 \times 26\);
24.32;
Award [2] for correct final answer.
Award [1 max] for 24.31 with correct working.
Award [0] for 24.31 (Data Booklet value) if working is incorrect or no working is shown.
Final answer must be to 2 decimal places to score [2].
Examiners report
In 2(a) the processes in the spectrometer were generally well described although many candidates did not mention that positive ions are formed. Relative atomic mass was defined poorly in (b)(i) but the atomic mass was generally calculated correctly. Most candidates gave their answers to the required two decimal places. Even though relative atomic mass was asked for, most candidates stated units for \({A_{\text{r}}}\).

