| Date | May 2014 | Marks available | 1 | Reference code | 14M.3.hl.TZ1.2 |
| Level | HL | Paper | 3 | Time zone | TZ1 |
| Command term | Show | Question number | 2 | Adapted from | N/A |
Question
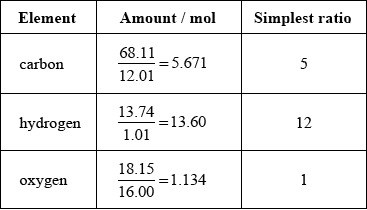
Organic compound X is 68.11% carbon, 13.74% hydrogen and 18.15 % oxygen by mass.
Show that the empirical formula of compound X is \({{\text{C}}_{\text{5}}}{{\text{H}}_{{\text{12}}}}{\text{O}}\).
The mass spectrum, infrared spectrum and details of the \(^{\text{1}}{\text{H}}\,{\text{NMR}}\) spectrum of compound X are given below.
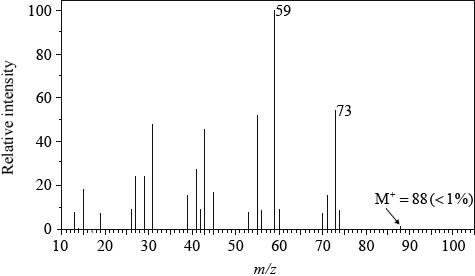
Mass spectrum:
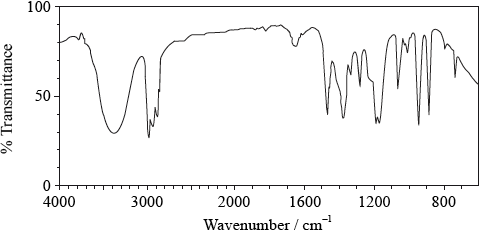
Infrared spectrum:
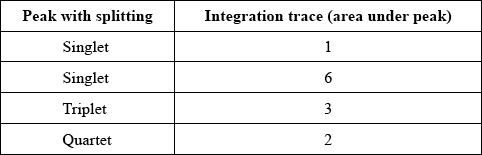
\(^{\text{1}}{\text{H}}\,{\text{NMR}}\) spectrum:
Analyse these three spectra and, using relevant information, deduce the identity of the compound.
Mass spectrum:
Infrared spectrum:
\(^{\text{1}}{\text{H}}\,{\text{NMR}}\) spectrum:
Identity of X:
Markscheme
 / OWTTE;
/ OWTTE;
Accept mass of C5H12O = 88.17 so % of \(C = \left( {\frac{{60.05}}{{88.17}}} \right) \times 100 = 68.11\),
% of \(H = \left( {\frac{{12.12}}{{88.17}}} \right) \times 100 = 13.75\) and % of \(O = \left( {\frac{{16.00}}{{88.17}}} \right) \times 100 = 18.15\).
Allow integer values for atomic masses.
Mass spectrum:
molecular ion peak at \({\text{88/}}{{\text{M}}^ + } = 88\) shows molecular formula is \({{\text{C}}_5}{{\text{H}}_{12}}{\text{O}}\);
absorption at 73 due to (M–CH3)+ / X contains a methyl group as peak at M–15 / OWTTE;
absorption at 59 due to (M–C2H5)+ / X contains an ethyl group as peak at M–29;
Penalise once only if + charge omitted.
Accept that X contains a CHO group due to M–29 but in fact it cannot as there are too many hydrogen atoms in the compound for it to be an aldehyde.
Infrared spectrum:
peak in range at 3200–3600 \({\text{c}}{{\text{m}}^{ - 1}}\) shows it contains an OH group / OWTTE;
(sharp) peaks just below \({\text{3000 c}}{{\text{m}}^{ - 1}}\)/in range 2850–3100 \({\text{c}}{{\text{m}}^{ - 1}}\) due to C–H
absorptions;
lack of peak at approximately \({\text{1700 c}}{{\text{m}}^{ - 1}}\) shows it does not contain C=O;
absorption between 1050 and \({\text{1410 c}}{{\text{m}}^{ - 1}}\) due to C–O;
Allow “due to alcohol” instead of due to C–O.
Accept “absorption between 1050 and 1410 cm–1 due to ether or ester” although it cannot be either as there is only one O atom and it has been identified as bonded to H.
fingerprint region specific to compound but needs to be compared with library / OWTTE;
1H NMR spectrum:
(12 protons are in) four different chemical environments (in the ratio 1:2:6:3);
singlet (with integration trace of 1) due to OH proton;
singlet (with integration trace of 6) suggests (two \({\text{C}}{{\text{H}}_{\text{3}}}\)) groups attached to a carbon atom with no Hs attached to it;
quartet (with integration trace of 2) due to \({\text{C}}{{\text{H}}_{\text{2}}}\) next to \({\text{C}}{{\text{H}}_{\text{3}}}\);
triplet (with integration trace of 3) due to \({\text{C}}{{\text{H}}_{\text{3}}}\) next to \({\text{C}}{{\text{H}}_{\text{2}}}\);
Reference must be made to the association of the splitting pattern (singlet, triplet etc.) to the specific carbon fragments.
(X is) 2-methylbutan-2-ol/\({\text{C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{C(C}}{{\text{H}}_{\text{3}}}{{\text{)}}_{\text{2}}}{\text{OH}}\);
No ECF throughout 2(b).
Examiners report
The question on empirical formula in (a) posed no difficulty and even the weaker candidates scored the mark. In the spectroscopy question in (b), some of the better candidates managed to score all 11 marks assigned to this extended response type question. In the MS, + was often omitted. Many students could not distinguish between observed ions and lost fragments. Only one candidate mentioned the "fingerprint" region of IR but did not refer to the need to compare with library spectral data. For the IR the majority of candidates scored all three marks, though the weaker candidates frequently suggested NH bonds and CF bonds even though neither nitrogen nor fluorine are part of the empirical formula given in the stem of the question. Discussion of the \(^{\text{1}}{\text{H}}\,{\text{NMR}}\) spectrum proved the most challenging and many candidates did not relate the splitting pattern to the specific carbon fragments. It was disappointing at HL seeing a number of candidates not including hydrogens in their final answer for the structural formula of 2-methylbutan-2-ol.
The question on empirical formula in (a) posed no difficulty and even the weaker candidates scored the mark. In the spectroscopy question in (b), some of the better candidates managed to score all 11 marks assigned to this extended response type question. In the MS, + was often omitted. Many students could not distinguish between observed ions and lost fragments. Only one candidate mentioned the "fingerprint" region of IR but did not refer to the need to compare with library spectral data. For the IR the majority of candidates scored all three marks, though the weaker candidates frequently suggested NH bonds and CF bonds even though neither nitrogen nor fluorine are part of the empirical formula given in the stem of the question. Discussion of the \(^{\text{1}}{\text{H}}\,{\text{NMR}}\) spectrum proved the most challenging and many candidates did not relate the splitting pattern to the specific carbon fragments. It was disappointing at HL seeing a number of candidates not including hydrogens in their final answer for the structural formula of 2-methylbutan-2-ol.

