| Date | November 2012 | Marks available | 1 | Reference code | 12N.2.hl.TZ0.7 |
| Level | HL | Paper | 2 | Time zone | TZ0 |
| Command term | Determine | Question number | 7 | Adapted from | N/A |
Question
Alkenes, alcohols and esters are three families of organic compounds with many commercial uses.
An ester which gives apples their characteristic smell contains C, H and O. When \(3.00 \times {10^{ - 3}}{\text{ g}}\) of this ester were completely combusted, \(6.93 \times {10^{ - 3}}{\text{ g}}\) of \({\text{C}}{{\text{O}}_{\text{2}}}\) and \(2.83 \times {10^{ - 3}}{\text{ g}}\) of \({{\text{H}}_{\text{2}}}{\text{O}}\) were produced.
State what is meant by the term stereoisomers.
Determine the empirical formula of the ester, showing your working.
The molar mass of the ester is \({\text{116.18 g}}\,{\text{mo}}{{\text{l}}^{ - 1}}\). Determine its molecular formula.
2-bromobutane is optically active. Draw the two enantiomers of 2-bromobutane and compare their physical and chemical properties.
Markscheme
compounds with same structural/displayed formula but different arrangements of atoms (in space);
Do not accept different 3D structures.
Do not allow similar instead of same.
Mass of C: \(\frac{{6.93 \times {{10}^{ - 3}}12.01}}{{44.01}} = 1.89 \times {10^{ - 3}}/0.00189{\text{ (g)}}\) and
Mass of H: \(\frac{{2 \times 1.01 \times 2.83 \times {{10}^{ - 3}}}}{{18.02}} = 3.17 \times {10^{ - 4}}/0.000317{\text{ (g)}}\);
Mass of O: \(3.00 \times {10^{ - 3}} - 1.89 \times {10^{ - 3}} - 3.17 \times {10^{ - 4}} = 7.93 \times {10^{ - 4}}/0.000793{\text{ (g)}}\);
\({n_C}:{\text{ }}\frac{{1.89 \times {{10}^{ - 3}}}}{{12.01}} = 1.57 \times {10^{ - 4}}/0.000157{\text{ (mol)}}\) and
\({n_H}:{\text{ }}\frac{{3.17 \times {{10}^{ - 4}}}}{{1.01}} = 3.14 \times {10^{ - 4}}/0.000314{\text{ (mol)}}\) and
\({n_O}:{\text{ }}\frac{{7.93 \times {{10}^{ - 4}}}}{{16.00}} = 4.96 \times {10^{ - 5}}/0.0000496{\text{ (mol)}}\);
Empirical formula \( = {{\text{C}}_3}{{\text{H}}_6}{\text{O}}\);
Allow C19H38O6.
Award [4] for correct final answer if alternative working is used.
Award [1 max] for C3H6O/C19H38O6 without working.
\({{\text{C}}_6}{{\text{H}}_{12}}{{\text{O}}_2}\);
Accept either one of the following two E2 mechanisms:
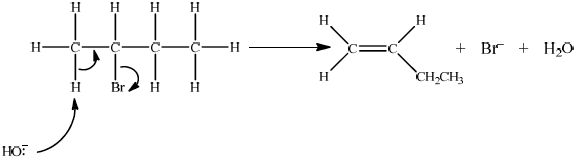
OR
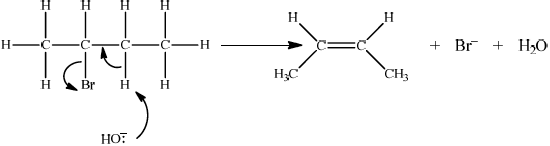
curly arrow going from lone pair/negative charge on O in \({\text{H}}{{\text{O}}^ - }\) to H on \(\beta \)–C;
Do not allow curly arrow originating on H in HO–.
curly arrow going from CH bond to form C=C bond;
curly arrow showing Br leaving;
formation of organic product \({{\text{H}}_2}{\text{C}}\)=\({\text{CH(C}}{{\text{H}}_2}{\text{C}}{{\text{H}}_3}{\text{)}}/{\text{H(C}}{{\text{H}}_3}{\text{)C}}\)=\({\text{CH(C}}{{\text{H}}_3}{\text{)}}\) and
\({\text{B}}{{\text{r}}^ - }\) and \({{\text{H}}_2}{\text{O}}\);
For this reaction since a strong negatively charged base, HO– is used, resultant mechanism will be E2. However, accept the corresponding E1 mechanism.
If E1, allow the following mechanism:
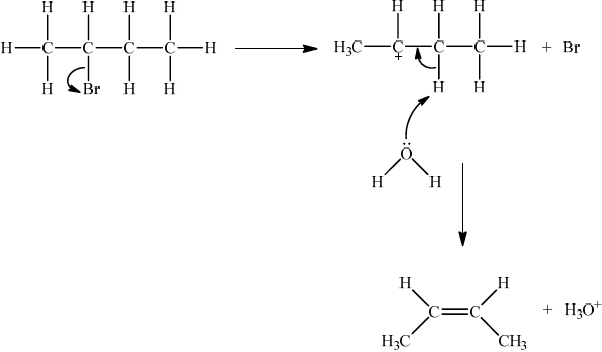
OR
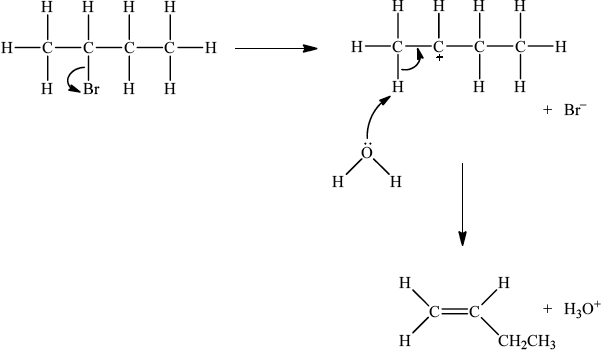
curly arrow showing Br leaving;
representation of secondary carbocation;
curly arrow going from lone pair on O in \({{\text{H}}_2}{\text{O}}\) to H on C adjacent to \({{\text{C}}^ + }\) and curly arrow going from CH bond to form C=C bond;
formation of organic product \({\text{(}}{{\text{H}}_3}{\text{C)CH}}\)=\({\text{CH(C}}{{\text{H}}_3}{\text{)}}\) / \({{\text{H}}_2}{\text{C}}\)=\({\text{CH(C}}{{\text{H}}_2}{\text{C}}{{\text{H}}_3}{\text{)}}\) and \({\text{B}}{{\text{r}}^ - }\) and \({{\text{H}}_3}{{\text{O}}^ + }\);
For E1 HO– is an alternative to H2O, but if used, H2O forms instead of H3O+.
Examiners report
This was the least popular question in Section B. In part (a) (i), some candidates gave a definition of structural isomers instead of stereoisomers.
Part (b) (i) proved to be very challenging for candidates. A large majority of candidates in fact did not know how to even commence the problem. There were a number of G2 comments all of who stated that it would have been better if the ratios of the amounts of C, H and O were in fact closer to whole number ratios.
In part (ii) of the question the molar mass of the ester was given as \({\text{116.18 g}}\,{\text{mo}}{{\text{l}}^{ - 1}}\), which meant that taking the experimental data given in (b) (i), the empirical formula is in fact \({{\text{C}}_{\text{3}}}{{\text{H}}_{\text{6}}}{\text{O}}\), with the associated molecular formula of \({{\text{C}}_{\text{6}}}{{\text{H}}_{{\text{12}}}}{{\text{O}}_{\text{2}}}\). The better students realised this and typically gave an answer of \({{\text{C}}_{\text{3}}}{{\text{H}}_{\text{6}}}{\text{O}}\). However, a very small minority did in fact use a scaling factor to suggest an empirical formula of \({{\text{C}}_{{\text{19}}}}{{\text{H}}_{{\text{38}}}}{{\text{O}}_{\text{6}}}\), which was also accepted. In general however for this question, candidates tended to score either scored full marks for parts (i) and (ii), or zero.
In part (iii), some candidates did not show the 3D nature of the two enantiomers which was necessary for M1 and only gave 2D representations. It was encouraging to see a greater percentage of candidates however using tapered (wedge/dash) representations. For M2, many did not mention the fact that the two optical isomers rotate the plane of polarized light in opposite directions. Some did not state plane.

