| Date | May 2009 | Marks available | 2 | Reference code | 09M.3.sl.TZ1.D1 |
| Level | SL | Paper | 3 | Time zone | TZ1 |
| Command term | State | Question number | D1 | Adapted from | N/A |
Question
Analgesics are used to relieve pain in the body. Aspirin and paracetamol (acetaminophen) are both mild analgesics.
The structures of the strong analgesics morphine and heroin (diamorphine) can be found in Table 20 of the Data Book
Compare how mild and strong analgesics relieve pain in the body.
Identify the amine functional group in the morphine molecule below by drawing a ring around it.
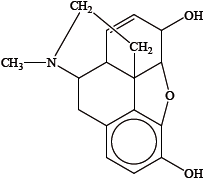
State the name of the functional group found in heroin but not in morphine.
State one advantage and one disadvantage of using morphine as a strong analgesic.
Markscheme
mild analgesics function by intercepting the pain stimulus at the source / interfere with the production of substances that cause pain/prostaglandins;
strong analgesics work by bonding to receptor sites in the brain / prevent the transmission of pain impulses without depressing the central nervous system;
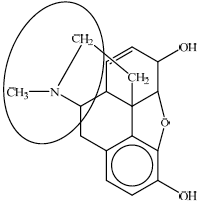
any circle around the nitrogen atom / the nitrogen atom and its three neighboring atoms;
ester;
Advantage: antidiarrheal/constipation (in treatment of diarrhea) / reduces coughing;
Disadvantage: addiction / tolerance / risk of overdose;
Examiners report
Most candidates were able to distinguish between the ways mild analgesics and strong analgesics relieve pain in part (b).
A substantial number of candidates failed to identify the tertiary amine in the structure of morphine. Candidates were inaccurate in drawing a circle around the amine group in part (c). Either just the nitrogen atom or nitrogen atom with its three neighbouring atoms should have been circled.
A large number of candidates confused the ester with an ether or carbonyl group as the functional group found in heroin but not in morphine.
Most candidates recognized the disadvantage of using morphine but they had extreme difficulty in stating a specific advantage for using morphine as a strong analgesic.

