| Date | November 2012 | Marks available | 1 | Reference code | 12N.2.sl.TZ0.5 |
| Level | SL | Paper | 2 | Time zone | TZ0 |
| Command term | State | Question number | 5 | Adapted from | N/A |
Question
Arsenic and nitrogen play a significant role in environmental chemistry. Arsenous acid, H3AsO3, can be found in oxygen-poor (anaerobic) water, and nitrogen-containing fertilizers can contaminate water.
Nitric acid, HNO3, is strong and nitrous acid, HNO2, is weak.
(i) Define oxidation and reduction in terms of electron loss or gain.
Oxidation:
Reduction:
(ii) Deduce the oxidation numbers of arsenic and nitrogen in each of the following species.
\({\text{A}}{{\text{s}}_{\text{2}}}{{\text{O}}_{\text{3}}}\):
\({\text{NO}}_3^ - \):
\({{\text{H}}_{\text{3}}}{\text{As}}{{\text{O}}_{\text{3}}}\):
\({{\text{N}}_{\text{2}}}{{\text{O}}_{\text{3}}}\):
(iii) Distinguish between the terms oxidizing agent and reducing agent.
(iv) In the removal of arsenic from contaminated groundwater, \({{\text{H}}_{\text{3}}}{\text{As}}{{\text{O}}_{\text{3}}}\) is often first oxidized to arsenic acid, \({{\text{H}}_{\text{3}}}{\text{As}}{{\text{O}}_{\text{4}}}\).
The following unbalanced redox reaction shows another method of forming \({{\text{H}}_{\text{3}}}{\text{As}}{{\text{O}}_{\text{4}}}\).
\[{\text{A}}{{\text{s}}_2}{{\text{O}}_3}{\text{(s)}} + {\text{NO}}_3^ - {\text{(aq)}} \to {{\text{H}}_3}{\text{As}}{{\text{O}}_4}{\text{(aq)}} + {{\text{N}}_2}{{\text{O}}_3}{\text{(aq)}}\]
Deduce the balanced redox equation in acid, and then identify both the oxidizing and reducing agents.
Define an acid according to the Brønsted–Lowry and Lewis theories.
Brønsted–Lowry theory:
Lewis theory:
The Lewis (electron dot) structure of nitrous acid is given below.
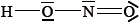
Identify which nitrogen-oxygen bond is the shorter.
Deduce the approximate value of the hydrogen-oxygen-nitrogen bond angle in nitrous acid and explain your answer.
Distinguish between a strong acid and a weak acid in terms of their dissociation in aqueous solution.
Ammonia, NH3, is a weak base. Deduce the Lewis (electron dot) structure of NH3. State the name of the shape of the molecule and explain why NH3 is a polar molecule.
When lime was added to a sample of soil, the pH changed from 5 to 7. Calculate the factor by which the hydrogen ion concentration changes.
One common nitrogen-containing fertilizer is ammonium sulfate. State its chemical formula.
Markscheme
(i) Oxidation: loss of electrons and Reduction: gain of electrons;
(ii) As2O3: +3;
NO3–: +5;
H3AsO3: +3;
N2O3: +3;
Penalize incorrect notation e.g. III, V, 3+, 5+, 3, 5 once only.
(iii) Oxidizing agent: substance reduced / removes electrons from another substance / causes some other substance to be oxidized / OWTTE and Reducing agent: substance oxidized / gives electrons to another substance / causes some other substance to be reduced / OWTTE;
Accept Oxidizing agent: electron/e/e– acceptor / causes oxidation / oxidation number/state decreases and Reducing agent: electron/e/e– donor / causes reduction / oxidation number/state increases.
(iv) \({\text{A}}{{\text{s}}_2}{{\text{O}}_3}{\text{(s)}} + {\text{2NO}}_3^ - {\text{(aq)}} + {\text{2}}{{\text{H}}^ + }{\text{(aq)}} + {\text{2}}{{\text{H}}_2}{\text{O(l)}} \to {\text{2}}{{\text{H}}_3}{\text{As}}{{\text{O}}_4}{\text{(aq)}} + {{\text{N}}_2}{{\text{O}}_3}{\text{(aq)}}\)
correct coefficients for \({\text{A}}{{\text{s}}_2}{{\text{O}}_3}\), \({{\text{H}}_3}{\text{As}}{{\text{O}}_4}\) and \({\text{NO}}_3^ - \), \({{\text{N}}_2}{{\text{O}}_3}\);
correct balanced equation;
Ignore state symbols.
M1 must be correct to award M2.
Oxidizing agent: \({\text{NO}}_3^ - {\text{(aq)}}\) / nitrate and Reducing agent: \({\text{A}}{{\text{s}}_2}{{\text{O}}_3}{\text{(s)}}\) / arsenic(III) oxide;
Accept HNO3(aq)/nitric acid.
Accept arsenic oxide.
Species must be fully correct to score M3.
Ignore state symbols.
Brønsted Lowry theory: proton/H+ donor;
Lewis theory: electron-pair acceptor;
N=O;
accept any value in range 102–105°;
Actual value is 102°.
lone/non-bonding pairs on oxygen occupy more space/repel more than bonding pairs hence decreasing the H–O–N bond angle (from 109.5° ) / OWTTE;
Strong acid: acid/electrolyte completely/100% dissociated/ionized in solution/water / OWTTE and Weak acid: acid/electrolyte partially dissociated/ionized in solution/water / OWTTE;
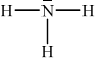 ;
;
Accept any combination of lines, dots or crosses to represent electron pairs.
trigonal/triangular pyramidal;
Accept pyramidal (since SL).
Do not allow tetrahedral.
net dipole moment present in molecule / NH bond polarities do not cancel each other out / unsymmetrical distribution of charge /OWTTE;
Do not accept molecule has no symmetry hence polar.
changes by 102 /100;
Allow changes from 10–5 to 10–7.
\({{\text{(N}}{{\text{H}}_4}{\text{)}}_2}{\text{S}}{{\text{O}}_4}\);
Examiners report
This was the most popular question answered in Section B.
The definition of oxidation and reduction, deducing oxidation numbers (although some forgot the + sign) and distinguishing between an oxidizing and reducing agent was answered very well by a majority of the candidates. However, a surprising number of candidates were unable to balance the redox equation or identify the correct oxidizing and reducing agents in the given reaction.
In part (b), most candidates defined an acid according to the Brønsted–Lowry and Lewis theories and identify the shorter bond in the Lewis structure given of \({\text{HN}}{{\text{O}}_{\text{2}}}\). Many candidates were able to deduce the approximate value of the H―O―N bond angle, however, some candidates were unable to explain in terms of the greater space occupied by the non-bonding electron pairs compared to the bonding electron pairs. Distinguishing between strong and weak acid in terms of their dissociation in aqueous solution was handled very well. The Lewis structure and shape of ammonia was done correctly by most candidates. However, the weaker candidates stated triangular planar instead of triangular pyramidal and that the molecule has no symmetry instead of unsymmetrical distribution of charge giving rise to a net dipole moment. The change in concentration with the change in pH was done well while an overwhelming number of candidates did not write the correct formula of ammonium sulphate.
In part (b), most candidates defined an acid according to the Brønsted–Lowry and Lewis theories and identify the shorter bond in the Lewis structure given of \({\text{HN}}{{\text{O}}_{\text{2}}}\). Many candidates were able to deduce the approximate value of the H―O―N bond angle, however, some candidates were unable to explain in terms of the greater space occupied by the non-bonding electron pairs compared to the bonding electron pairs. Distinguishing between strong and weak acid in terms of their dissociation in aqueous solution was handled very well. The Lewis structure and shape of ammonia was done correctly by most candidates. However, the weaker candidates stated triangular planar instead of triangular pyramidal and that the molecule has no symmetry instead of unsymmetrical distribution of charge giving rise to a net dipole moment. The change in concentration with the change in pH was done well while an overwhelming number of candidates did not write the correct formula of ammonium sulphate.
In part (b), most candidates defined an acid according to the Brønsted–Lowry and Lewis theories and identify the shorter bond in the Lewis structure given of \({\text{HN}}{{\text{O}}_{\text{2}}}\). Many candidates were able to deduce the approximate value of the H―O―N bond angle, however, some candidates were unable to explain in terms of the greater space occupied by the non-bonding electron pairs compared to the bonding electron pairs. Distinguishing between strong and weak acid in terms of their dissociation in aqueous solution was handled very well. The Lewis structure and shape of ammonia was done correctly by most candidates. However, the weaker candidates stated triangular planar instead of triangular pyramidal and that the molecule has no symmetry instead of unsymmetrical distribution of charge giving rise to a net dipole moment. The change in concentration with the change in pH was done well while an overwhelming number of candidates did not write the correct formula of ammonium sulphate.
In part (b), most candidates defined an acid according to the Brønsted–Lowry and Lewis theories and identify the shorter bond in the Lewis structure given of \({\text{HN}}{{\text{O}}_{\text{2}}}\). Many candidates were able to deduce the approximate value of the H―O―N bond angle, however, some candidates were unable to explain in terms of the greater space occupied by the non-bonding electron pairs compared to the bonding electron pairs. Distinguishing between strong and weak acid in terms of their dissociation in aqueous solution was handled very well. The Lewis structure and shape of ammonia was done correctly by most candidates. However, the weaker candidates stated triangular planar instead of triangular pyramidal and that the molecule has no symmetry instead of unsymmetrical distribution of charge giving rise to a net dipole moment. The change in concentration with the change in pH was done well while an overwhelming number of candidates did not write the correct formula of ammonium sulphate.
In part (b), most candidates defined an acid according to the Brønsted–Lowry and Lewis theories and identify the shorter bond in the Lewis structure given of \({\text{HN}}{{\text{O}}_{\text{2}}}\). Many candidates were able to deduce the approximate value of the H―O―N bond angle, however, some candidates were unable to explain in terms of the greater space occupied by the non-bonding electron pairs compared to the bonding electron pairs. Distinguishing between strong and weak acid in terms of their dissociation in aqueous solution was handled very well. The Lewis structure and shape of ammonia was done correctly by most candidates. However, the weaker candidates stated triangular planar instead of triangular pyramidal and that the molecule has no symmetry instead of unsymmetrical distribution of charge giving rise to a net dipole moment. The change in concentration with the change in pH was done well while an overwhelming number of candidates did not write the correct formula of ammonium sulphate.
In part (b), most candidates defined an acid according to the Brønsted–Lowry and Lewis theories and identify the shorter bond in the Lewis structure given of \({\text{HN}}{{\text{O}}_{\text{2}}}\). Many candidates were able to deduce the approximate value of the H―O―N bond angle, however, some candidates were unable to explain in terms of the greater space occupied by the non-bonding electron pairs compared to the bonding electron pairs. Distinguishing between strong and weak acid in terms of their dissociation in aqueous solution was handled very well. The Lewis structure and shape of ammonia was done correctly by most candidates. However, the weaker candidates stated triangular planar instead of triangular pyramidal and that the molecule has no symmetry instead of unsymmetrical distribution of charge giving rise to a net dipole moment. The change in concentration with the change in pH was done well while an overwhelming number of candidates did not write the correct formula of ammonium sulphate.
In part (b), most candidates defined an acid according to the Brønsted–Lowry and Lewis theories and identify the shorter bond in the Lewis structure given of \({\text{HN}}{{\text{O}}_{\text{2}}}\). Many candidates were able to deduce the approximate value of the H―O―N bond angle, however, some candidates were unable to explain in terms of the greater space occupied by the non-bonding electron pairs compared to the bonding electron pairs. Distinguishing between strong and weak acid in terms of their dissociation in aqueous solution was handled very well. The Lewis structure and shape of ammonia was done correctly by most candidates. However, the weaker candidates stated triangular planar instead of triangular pyramidal and that the molecule has no symmetry instead of unsymmetrical distribution of charge giving rise to a net dipole moment. The change in concentration with the change in pH was done well while an overwhelming number of candidates did not write the correct formula of ammonium sulphate.

