| Date | May 2013 | Marks available | 1 | Reference code | 13M.2.sl.TZ2.5 |
| Level | SL | Paper | 2 | Time zone | TZ2 |
| Command term | State | Question number | 5 | Adapted from | N/A |
Question
Ionic bonding and covalent bonding are two types of bonding.
Consider the molecules sulfur difluoride, \({\text{S}}{{\text{F}}_{\text{2}}}\), boron trifluoride, \({\text{B}}{{\text{F}}_{\text{3}}}\), and phosphorus trichloride, \({\text{PC}}{{\text{l}}_{\text{3}}}\).
Ionic bonding occurs in sodium chloride. Describe what is meant by the term ionic bonding.
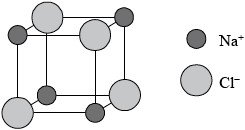
Sodium chloride has a lattice structure. Describe the lattice structure of sodium chloride including a suitable representative three-dimensional diagram. On the diagram, label each ion and distinguish between the different types of ions present using different sized spheres.
Ammonium phosphate is also an ionic compound, used in the manufacture of fertilizers. State the chemical formula of ammonium phosphate.
Deduce the Lewis (electron dot) structure and predict the shape of each molecule, using the valence shell electron pair repulsion theory (VSEPR).
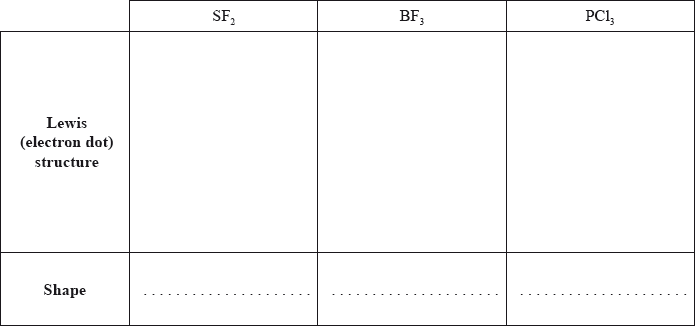
State and explain the F–S–F bond angle in SF2.
Deduce whether each of the three molecules is polar or non-polar, giving your reason in each case.
SF2:
BF3:
PCl3:
Using electronegativity values from Table 7 of the Data Booklet, state and explain which of the following compounds, IBr, BaCl2, CsI and HBr are ionic and which compounds are covalent.
IBr:
BaCl2:
CsI:
HBr:
Markscheme
(electrostatic) attraction between oppositely charged ions/cation and anion/positive and negative ions;
Do not allow electrostatic attraction between metals and non-metals.
Description:
a lattice is a giant, regular/repeating arrangement/array;
of (chloride) anions/negative ions/\({\text{C}}{{\text{l}}^ - }\) and (sodium) cations/positive ions/\({\text{N}}{{\text{a}}^ + }\);
each sodium ion surrounded by six chloride ions / each chloride ion surrounded by six sodium ions;
M2 may also be scored from a diagrammatical key or labels on each ion.
M3 may also be scored by a correctly represented cubic representation showing the six-coordination around either the sodium ion or each chloride ion.
Diagram:
cubic lattice type representation (showing a minimum of one sub-cube and alternating \({\text{N}}{{\text{a}}^ + }\) and \({\text{C}}{{\text{l}}^ - }\) ions);
\({\text{C}}{{\text{l}}^ - }\) shown represented bigger than \({\text{N}}{{\text{a}}^ + }\) on diagram;
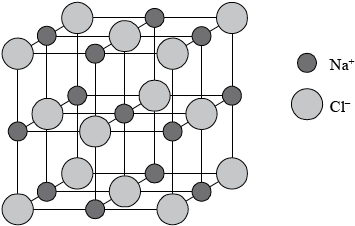
Award [4] for correctly drawn diagram (like above) with ions clearly identified.
Award [3 max] for the following diagram below if no explanation in words is given.
(NH4)3PO4;
Allow use of square brackets.
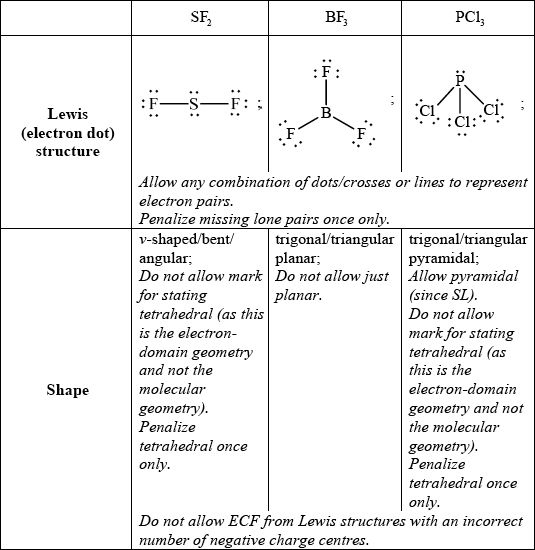
allow any bond angle in the range 97° to less than 109.5° (experimental value is 98°);
due to four negative charge centres/four electron pairs/four electron domains (two of which are lone pairs)/tetrahedral arrangement of electron pairs;
extra repulsion due to two lone pairs of electrons repelling each other / lone pairs occupy more space (than bonding pairs) so F–S–F bond angle decreases from 109.5° / OWTTE;
Answers which refer to electronegativity consideration of F’s also are correct, as long as LP/LP repulsion is also mentioned to score M3.
SF2:
polar because net dipole moment present in molecule / SF bond polarities do not cancel each other out / unsymmetrical distribution of charge / OWTTE;
BF3:
non-polar because no net dipole moment present in molecule / BF bond polarities do cancel each other out / symmetrical distribution of charge / OWTTE;
PCl3:
polar because net dipole moment present in molecule / PCl bond polarities do not cancel each other out / unsymmetrical distribution of charge / OWTTE;
Award [1 max] for SF2 polar, BF3 non-polar, PCl3 polar even if explanations are incorrect or are not given.
Polarity may also be explained using diagrams showing net dipole moments.
IBr:
\(\Delta \chi = (3.0 - 2.7) = 0.3\), covalent
BaCl2:
\(\Delta \chi = (3.2 - 0.9) = 2.3\), ionic
CsI:
\(\Delta \chi = (2.7 - 0.8) = 1.9\), ionic
HBr:
\(\Delta \chi = (3.0 - 2.2) = 0.8\), covalent
Award [2] for all four correct, [1] for two or three correct.
Award [1 max] for stating IBr, HBr covalent and BaCl2, CsI ionic.
Allow polar covalent instead of covalent.
Allow large electronegativity difference for ionic and small electronegativity difference for covalent.
Examiners report
Many candidates failed to score for the meaning of the term ionic bonding in a) (i). A definition should provide an easily scored mark.
Part a) (ii) required a description and a diagram of a sodium chloride lattice. Marks were awarded so that a candidate who attempted a diagram and gave a good description could score full marks.
In a) (iii) the chemical formula of ammonium phosphate was sometimes creatively constructed with amm used as the symbol for the ammonium ion and phosphate as \({\text{P}}{{\text{O}}_{\text{3}}}\) or as \({\text{PO}}_{\text{4}}^{2 - }\).
Lewis structures in b) (i) were generally well done. The most common loss of a mark was due to omitting lone pairs of electrons from atoms.
In b) (ii) many candidates stated that S had two lone pairs of electrons but still based the bond angle on a trigonal planar structure. Even candidates who correctly stated the bond angle could not explain it well.
In b) (iii) many candidates could identify the molecules as polar or non-polar but could not give a valid reason. Some referred to charges cancelling out rather than dipoles.
Part c) required candidates to find differences in electronegativity values to determine if compounds are ionic or covalent. Many candidates answered this well. Some found the electronegativity difference correctly but were confused about how to use this to classify the type of bonding present. A few candidates added the electronegativity values.

