| Date | May 2014 | Marks available | 1 | Reference code | 14M.1.sl.TZ2.10 |
| Level | SL | Paper | 1 | Time zone | TZ2 |
| Command term | Question number | 10 | Adapted from | N/A |
Question
Which properties do typical ionic compounds have?
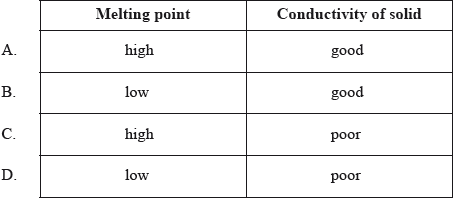
Markscheme
C
Examiners report
[N/A]
Syllabus sections
Show 66 related questions
- 17M.2.sl.TZ2.1c.ii: Explain the electrical conductivity of molten Na2O and P4O10.
- 17M.1.sl.TZ2.19: Which of the following does not react with dilute HCl(aq)? A. Na2CO3 B. Cu C. ...
- 17M.1.sl.TZ2.10: Which bonds cause the boiling point of water to be significantly greater than that of...
- 17M.3.sl.TZ1.6b: Lanthanum has a similar electronegativity to group 2 metals. Explain, in terms of bonding and...
- 17M.2.sl.TZ1.2e.i: State the type of bonding in potassium chloride which melts at 1043 K.
- 17M.1.sl.TZ1.9: A substance has the following properties: What is the most probable structure of this...
- 16N.3.hl.TZ0.22c: (i) Uranium hexafluoride, UF6, is used in the uranium enrichment process that produces fuel...
- 16N.2.sl.TZ0.4f: Describe the structure and bonding in solid magnesium oxide.
- 16M.1.sl.TZ0.10: Which compound contains both ionic and covalent bonds? A. SIH4 B. NaNO3 C. H2CO D. Na2S
- 15M.1.hl.TZ1.11: Which substance has the following properties? • Low melting point • Very soluble in...
- 15M.2.hl.TZ1.2b.ii: Predict whether phosphorus(V) oxide and sodium oxide conduct electricity in their solid and...
- 15M.2.hl.TZ1.2b.i: Explain why the melting point of phosphorus(V) oxide is lower than that of sodium oxide in...
- 15M.2.hl.TZ2.8b.ii: Describe the ionic bonding present in...
- 15M.2.hl.TZ2.8b.iii: Suggest why solid \({\text{C}}{{\text{r}}_{\text{2}}}{{\text{O}}_{\text{3}}}\) does not...
- 15M.2.hl.TZ2.9a.i: State and explain the electrical conductivities of these two chloride compounds in their...
- 15M.3.hl.TZ1.17b.i: Phosphate ions can be removed from a solution by adding calcium ions. State the ionic...
- 15M.1.sl.TZ2.9: The formula of gallium phosphate is \({\text{GaP}}{{\text{O}}_{\text{4}}}\). What is the...
- 15M.2.sl.TZ1.6f.i: Describe the bonding and structure of sodium chloride.
- 15M.2.sl.TZ1.6f.ii: State the formula of the compounds formed between the elements below. Sodium and...
- 15M.2.sl.TZ2.3b: Outline why solid magnesium chloride does not conduct electricity.
- 15M.2.sl.TZ2.6b.iv: Describe the ionic bonding present in potassium chloride and how the ions are formed.
- 14M.1.hl.TZ1.11: A solid has a melting point of 1582 °C and does not dissolve in water. It does not conduct...
- 14M.2.hl.TZ1.5a: (i) State the changes in the acid-base nature of the oxides across period 3 (from...
- 14M.1.sl.TZ2.9: What is the formula of calcium phosphide? A. ...
- 14N.2.hl.TZ0.8c: (i) Magnesium reacts with oxygen to form an ionic compound, magnesium oxide. Describe how...
- 13N.1.hl.TZ0.9: What is the formula of calcium nitride? A. ...
- 13N.1.hl.TZ0.10: Which compounds have an ionic lattice structure in the solid state? I. Silicon...
- 13N.2.hl.TZ0.3a.i: State the formula of both ions present and the nature of the force between these...
- 13N.1.sl.TZ0.10: What is the formula of calcium nitride? A. ...
- 13N.1.sl.TZ0.11: Which compounds have an ionic lattice structure in the solid state? I. Silicon...
- 13N.2.sl.TZ0.3a.i: State the formula of both ions present and the nature of the force between these...
- 13M.1.sl.TZ1.13: Which combination best describes the type of bonding present and the melting point of silicon...
- 13M.1.sl.TZ1.10: Which statement best describes ionic bonding? A. It is the electrostatic attraction...
- 13M.1.sl.TZ1.12: Which statements concerning the sodium chloride ionic lattice are correct? I. Sodium...
- 13M.1.sl.TZ1.11: What are the correct formulas of the following ions?
- 13M.2.sl.TZ1.3a: Compare how electric current passes through sodium and sodium chloride by completing the...
- 13M.2.sl.TZ2.5a.i: Ionic bonding occurs in sodium chloride. Describe what is meant by the term ionic bonding.
- 13M.2.sl.TZ2.5a.ii: Sodium chloride has a lattice structure. Describe the lattice structure of sodium chloride...
- 13M.2.sl.TZ2.5a.iii: Ammonium phosphate is also an ionic compound, used in the manufacture of fertilizers. State...
- 13M.2.sl.TZ2.5c: Using electronegativity values from Table 7 of the Data Booklet, state and explain which of...
- 12N.1.sl.TZ0.10: What is the formula of the ionic compound formed when calcium and nitrogen react...
- 12N.1.sl.TZ0.13: Which statement about the physical properties of substances is correct? A. The only...
- 12N.2.sl.TZ0.4c: (i) Explain why metals are good conductors of electricity and why they are...
- 12N.2.sl.TZ0.5b.vii: One common nitrogen-containing fertilizer is ammonium sulfate. State its chemical formula.
- 09N.1.sl.TZ0.10: What compound is formed when lithium reacts with selenium? A. LiSe B. ...
- 09N.1.sl.TZ0.14: Which substance does not conduct electricity? A. Solid zinc B. Molten zinc C. ...
- 10M.1.sl.TZ2.10: What is the formula of magnesium fluoride? A. ...
- 09M.1.sl.TZ1.11: What are the correct formulas of the following ions?
- 09M.1.sl.TZ1.13: Which is the best description of ionic bonding? A. The electrostatic attraction between...
- 09M.2.sl.TZ1.3a: Explain why solid sodium oxide is a non-conductor of electricity.
- 09M.1.sl.TZ2.10: Which statement best describes the intramolecular bonding in HCN(l)? A. Electrostatic...
- 09M.1.sl.TZ2.12: Metal M has only one oxidation number and forms a compound with the formula...
- 09M.2.sl.TZ2.5b.iii: Explain why solid sodium chloride does not conduct electricity but molten sodium chloride does.
- 11M.2.hl.TZ1.6f.i: Explain the electrical conductivity of molten sodium oxide and liquid sulfur trioxide.
- 11M.1.sl.TZ1.13: Which particles are responsible for electrical conductivity in metals? A. Anions B. ...
- 11M.1.sl.TZ1.12: Which combination of the characteristics of element X, a metal, and element Y, a non metal,...
- 11M.1.sl.TZ2.12: The number of electrons in the valence shell of elements A and B, are 6 and 7 respectively....
- 11M.1.sl.TZ2.14: Which particles are responsible for the conduction of electricity in molten aluminium? A. ...
- 12M.2.hl.TZ2.7c.i: Aluminium chloride, \({\text{A}}{{\text{l}}_{\text{2}}}{\text{C}}{{\text{l}}_{\text{6}}}\),...
- 12M.1.sl.TZ2.10: What is the formula of magnesium nitride? A. ...
- 11N.2.hl.TZ0.6b.i: State and explain the difference in the electrical conductivity in the liquid state of the...
- 11N.1.sl.TZ0.9: What are the correct formulas of the following ions?
- 11N.2.sl.TZ0.1a: Sodium azide involves ionic bonding, and metallic bonding is present in sodium. Describe...
- 11N.2.sl.TZ0.5d.i: State the name of A.
- 11N.1.sl.TZ0.10: Which row correctly describes the bonding type and melting point of carbon and carbon dioxide?
- 11N.2.sl.TZ0.3b: Explain why solid sodium chloride does not conduct electricity.

