| Date | November 2014 | Marks available | 1 | Reference code | 14N.2.sl.TZ0.1 |
| Level | SL | Paper | 2 | Time zone | TZ0 |
| Command term | Calculate | Question number | 1 | Adapted from | N/A |
Question
A student used a pH meter to measure the pH of different samples of water at 298 K.
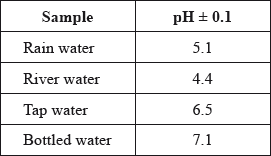
Use the data in the table to identify the most acidic water sample.
Calculate the percentage uncertainty in the measured pH of the rain water sample.
Determine the ratio of \({\text{[}}{{\text{H}}^ + }{\text{]}}\) in bottled water to that in rain water.
\[\frac{{[{H^ + }]{\text{ }}in{\text{ }}bottled{\text{ }}water}}{{[{H^ + }]{\text{ }}in{\text{ }}rain{\text{ }}water}}\]
The acidity of non-polluted rain water is caused by dissolved carbon dioxide. State an equation for the reaction of carbon dioxide with water.
Markscheme
river (water);
\(\left( {\frac{{0.1}}{{5.1}} \times 100 = } \right){\text{ }}2\% \);
recognition that values differ by 2 Ph units / calculation of both \({\text{[}}{{\text{H}}^ + }{\text{]}}\) values;
\({\text{(ratio)}} = 1:100/{10^{ - 2}}/0.01/\frac{1}{{100}}\);
Award [2] for correct final answer.
Award [1 max] for 100:1/100/102.
\({\text{C}}{{\text{O}}_2} + {{\text{H}}_2}{\text{O}} \rightleftharpoons {\text{HCO}}_3^ - + {{\text{H}}^ + }/{\text{C}}{{\text{O}}_2} + {\text{2}}{{\text{H}}_2}{\text{O}} \rightleftharpoons {\text{HCO}}_3^ - + {{\text{H}}_3}{{\text{O}}^ + }/{\text{C}}{{\text{O}}_2} + {{\text{H}}_2}{\text{O}} \rightleftharpoons {{\text{H}}_2}{\text{C}}{{\text{O}}_3}\);
Do not penalize missing reversible arrow.
Do not accept equations with the carbonate ion as a product.
Examiners report
Parts (a) and (b) were correctly answered by the majority of candidates, the most common mistake being to assume that (b) referred to the sample identified in (a). Part (c) was rather more challenging and students frequently used the ratio of the pH rather than the ratio of the \({\text{[}}{{\text{H}}^ + }{\text{]}}\). Part (d) should have been very straightforward, but was often poorly answered with some innovative products. The absence of an equilibrium arrow was not penalised, but if it had been many students would have lost a mark.
Parts (a) and (b) were correctly answered by the majority of candidates, the most common mistake being to assume that (b) referred to the sample identified in (a). Part (c) was rather more challenging and students frequently used the ratio of the pH rather than the ratio of the \({\text{[}}{{\text{H}}^ + }{\text{]}}\). Part (d) should have been very straightforward, but was often poorly answered with some innovative products. The absence of an equilibrium arrow was not penalised, but if it had been many students would have lost a mark.
Parts (a) and (b) were correctly answered by the majority of candidates, the most common mistake being to assume that (b) referred to the sample identified in (a). Part (c) was rather more challenging and students frequently used the ratio of the pH rather than the ratio of the \({\text{[}}{{\text{H}}^ + }{\text{]}}\). Part (d) should have been very straightforward, but was often poorly answered with some innovative products. The absence of an equilibrium arrow was not penalised, but if it had been many students would have lost a mark.
Parts (a) and (b) were correctly answered by the majority of candidates, the most common mistake being to assume that (b) referred to the sample identified in (a). Part (c) was rather more challenging and students frequently used the ratio of the pH rather than the ratio of the \({\text{[}}{{\text{H}}^ + }{\text{]}}\). Part (d) should have been very straightforward, but was often poorly answered with some innovative products. The absence of an equilibrium arrow was not penalised, but if it had been many students would have lost a mark.

