| Date | May 2015 | Marks available | 1 | Reference code | 15M.2.sl.TZ1.1 |
| Level | SL | Paper | 2 | Time zone | TZ1 |
| Command term | State | Question number | 1 | Adapted from | N/A |
Question
Ethanedioic acid is a diprotic acid. A student determined the value of x in the formula of hydrated ethanedioic acid, \({\text{HOOC}}\)–\({\text{COOH}} \bullet {\text{x}}{{\text{H}}_{\text{2}}}{\text{O}}\), by titrating a known mass of the acid with a \({\text{0.100 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\) solution of \({\text{NaOH(aq)}}\).
0.795 g of ethanedioic acid was dissolved in distilled water and made up to a total volume of \({\text{250 c}}{{\text{m}}^{\text{3}}}\) in a volumetric flask.
\({\text{25 c}}{{\text{m}}^{\text{3}}}\) of this ethanedioic acid solution was pipetted into a flask and titrated against aqueous sodium hydroxide using phenolphthalein as an indicator.
The titration was then repeated twice to obtain the results below.
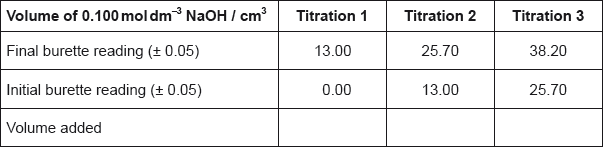
State the uncertainty of the volume of NaOH added in \({\text{c}}{{\text{m}}^{\text{3}}}\).
Calculate the average volume of NaOH added, in \({\text{c}}{{\text{m}}^{\text{3}}}\), in titrations 2 and 3, and then calculate the amount, in mol, of NaOH added.
(i) The equation for the reaction taking place in the titration is:
\({\text{HOOC}}\)−\({\text{COOH(aq)}} + {\text{2NaOH(aq)}} \to {\text{NaOOC}}\)−\({\text{COONa(aq)}} + {\text{2}}{{\text{H}}_{\text{2}}}{\text{O(l)}}\)
Determine the amount, in mol, of ethanedioic acid that reacts with the average volume of \({\text{NaOH(aq)}}\).
(ii) Determine the amount, in mol, of ethanedioic acid present in \({\text{250 c}}{{\text{m}}^{\text{3}}}\) of the original solution.
(ii) Determine the molar mass of hydrated ethanedioic acid.
(iv) Determine the value of x in the formula \({\text{HOOC}}\)−\({\text{COOH}} \bullet {\text{x}}{{\text{H}}_{\text{2}}}{\text{O}}\).
Identify the strongest intermolecular force in solid ethanedioic acid.
Deduce the Lewis (electron dot) structure of ethanedioic acid, HOOC−COOH.
Markscheme
\(( \pm )0.10{\text{ (c}}{{\text{m}}^{\text{3}}}{\text{)}}\);
Accept ±0.1 (cm3).
Accept (±)0.09 (cm3) (based on more accurate method of calculating propagation of uncertainties).
\(\left( {\frac{{12.70 + 12.50}}{2} = } \right)12.60{\text{ (c}}{{\text{m}}^{\text{3}}}{\text{)}}\);
\((0.01260 \times 0.100 = )1.26 \times {10^{ - 3}}{\text{ (mol)}}\);
Award [2] for correct final answer.
(i) \(\left( {\frac{{1.26 \times {{10}^{ - 3}}}}{2} = } \right)6.30 \times {10^{ - 4}}{\text{ (mol)}}\);
(ii) \((6.30 \times {10^{ - 4}} \times 10 = )6.30 \times {10^{ - 3}}{\text{ (mol)}}\);
(iii) \(\left( {\frac{{0.795}}{{6.30 \times {{10}^{ - 3}}}} = } \right)126{\text{ (gmo}}{{\text{l}}^{ - 1}})\);
(iv) \({M_{\text{r}}}{\text{(}}{{\text{C}}_2}{{\text{H}}_2}{{\text{O}}_4}{\text{)}} = 90.04\) and \({M_{\text{r}}}{\text{(}}{{\text{H}}_2}{\text{O)}} = 18.02\);
\({\text{x}} = 2\);
Accept integer values for Mr’s of 90 and 18 and any reasonable calculation.
Award [1 max] if no working shown.
hydrogen bonding;
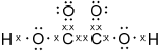 ;
;
Mark cannot be scored if lone pairs are missing on oxygens.
Accept any combination of lines, dots or crosses to represent electron pairs.
Examiners report
This beginning of this question to state the uncertainty and to calculate the average volume added were well done and most students could also calculate the number of moles added. However, many candidates began to lose marks from this point onwards. Some could identify the ratio and correctly state the moles of ethanedioic acid, but fewer realized they needed to multiply 10 to get back to the original solution. The next step to calculate the \({M_{\text{r}}}\) was only correctly completed by a handful of students. Those that were correct with the molar mass always could calculate the moles of water, many students just guessed an answer though.
The intermolecular force was correctly described as hydrogen bonding, however there were some instances when it seemed unclear whether students realized this was between molecules and instead they seemed to suggest it was a bond between hydrogen and oxygen in the molecule. Some candidates could correctly draw the Lewis structure but a number of those lost marks for omitting the lone pairs on oxygen.
This beginning of this question to state the uncertainty and to calculate the average volume added were well done and most students could also calculate the number of moles added. However, many candidates began to lose marks from this point onwards. Some could identify the ratio and correctly state the moles of ethanedioic acid, but fewer realized they needed to multiply 10 to get back to the original solution. The next step to calculate the \({M_{\text{r}}}\) was only correctly completed by a handful of students. Those that were correct with the molar mass always could calculate the moles of water, many students just guessed an answer though.
The intermolecular force was correctly described as hydrogen bonding, however there was some instances when it seemed unclear whether students realized this was between molecules and instead they seemed to suggest it was a bond between hydrogen and oxygen in the molecule. Some candidates could correctly draw the Lewis structure but a number of those lost marks for omitting the lone pairs on oxygen.
This beginning of this question to state the uncertainty and to calculate the average volume added were well done and most students could also calculate the number of moles added. However, many candidates began to lose marks from this point onwards. Some could identify the ratio and correctly state the moles of ethanedioic acid, but fewer realized they needed to multiply 10 to get back to the original solution. The next step to calculate the \({M_{\text{r}}}\) was only correctly completed by a handful of students. Those that were correct with the molar mass always could calculate the moles of water, many students just guessed an answer though.
The intermolecular force was correctly described as hydrogen bonding, however there was some instances when it seemed unclear whether students realized this was between molecules and instead they seemed to suggest it was a bond between hydrogen and oxygen in the molecule. Some candidates could correctly draw the Lewis structure but a number of those lost marks for omitting the lone pairs on oxygen.
This beginning of this question to state the uncertainty and to calculate the average volume added were well done and most students could also calculate the number of moles added. However, many candidates began to lose marks from this point onwards. Some could identify the ratio and correctly state the moles of ethanedioic acid, but fewer realized they needed to multiply 10 to get back to the original solution. The next step to calculate the \({M_{\text{r}}}\) was only correctly completed by a handful of students. Those that were correct with the molar mass always could calculate the moles of water, many students just guessed an answer though.
The intermolecular force was correctly described as hydrogen bonding, however there was some instances when it seemed unclear whether students realized this was between molecules and instead they seemed to suggest it was a bond between hydrogen and oxygen in the molecule. Some candidates could correctly draw the Lewis structure but a number of those lost marks for omitting the lone pairs on oxygen.
This beginning of this question to state the uncertainty and to calculate the average volume added were well done and most students could also calculate the number of moles added. However, many candidates began to lose marks from this point onwards. Some could identify the ratio and correctly state the moles of ethanedioic acid, but fewer realized they needed to multiply 10 to get back to the original solution. The next step to calculate the \({M_{\text{r}}}\) was only correctly completed by a handful of students. Those that were correct with the molar mass always could calculate the moles of water, many students just guessed an answer though.
The intermolecular force was correctly described as hydrogen bonding, however there was some instances when it seemed unclear whether students realized this was between molecules and instead they seemed to suggest it was a bond between hydrogen and oxygen in the molecule. Some candidates could correctly draw the Lewis structure but a number of those lost marks for omitting the lone pairs on oxygen.

