| Date | November 2014 | Marks available | 1 | Reference code | 14N.2.hl.TZ0.2 |
| Level | HL | Paper | 2 | Time zone | TZ0 |
| Command term | Predict | Question number | 2 | Adapted from | N/A |
Question
The reaction between ethene and steam is used in the industrial production of ethanol.
\[{{\text{C}}_{\text{2}}}{{\text{H}}_{\text{4}}}{\text{(g)}} + {{\text{H}}_{\text{2}}}{\text{O(g)}} \to {{\text{C}}_{\text{2}}}{{\text{H}}_{\text{5}}}{\text{OH(g)}}\]
The enthalpy change of the reaction can be calculated either by using average bond enthalpies or by using standard enthalpies of formation.
Determine the enthalpy change of the reaction, in \({\text{kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}\), using the average bond enthalpies in Table 10 of the Data Booklet.
(i) Define the term standard enthalpy change of formation.
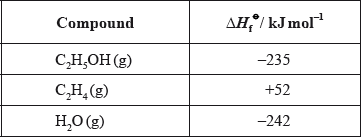
(ii) Determine the enthalpy change of the reaction, in \({\text{kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}\), between ethene and steam using the enthalpy change of formation values given below.
Comment on which of the values obtained in (a) and (b)(ii) is more accurate, giving a reason.
Predict the sign of the entropy change of the reaction, \(\Delta S\), giving a reason.
Markscheme
(bonds broken) C=C and O–H / 612 + 464 / 1076;
(bonds formed) C–C and C–H and C–O / 347 + 413 + 358 / 1118;
OR
(bonds broken) C=C and two O–H and four C–H / 612 + 4(413) + 2(464) / 3192;
(bonds formed) C–C and five C–H and C–O and O–H / 347 + 5(413) + 358 + 464 / 3234;
Ignore signs (+ and –) in M1 and M2. These two marks are awarded for recognizing the correct bonds.
enthalpy change \( = - 42{\text{ (kJ)}}\);
Correct sign is necessary for awarding M3.
Award [3] for the correct final answer.
Do not penalize candidates using the former Data Booklet bond energy values (348, 412 and 463) (final answer will then be –45(kJ)).
(i) heat/enthalpy change when 1 mol of a compound/substance is formed;
from its elements in their standard states/at \({\text{100 kPa/1}}{{\text{0}}^{\text{5}}}{\text{ Pa}}\);
Allow 1.01 \( \times \) 105 Pa/101 kPa/1 atm as an alternative to 100 kPa/105 Pa.
Allow under standard conditions or standard ambient temperature and pressure as an alternative to 100 kPa/105 Pa.
Allow “energy needed/absorbed” as an alternative to “heat/enthalpy change”.
Temperature is not required in definition, allow if quoted (eg, 298 K / 25 °C).
(ii) \(( - 235) - (52 - 242)/\Delta H = \Sigma \Delta H_{\text{f}}^\Theta {\text{(products)}} - \Sigma \Delta H_{\text{f}}^\Theta {\text{(reactants)}}\);
–45 (kJ);
Award [2] for the correct final answer.
Award [1] for +45 or 45.
value in (b)(ii) (is more accurate) as values used in (a) are average values / value in (b)(ii) (is more accurate) as exact bond enthalpy depends on the surroundings of the bond / OWTTE;
negative and fewer number of moles/molecules (of gas);
Examiners report
More than half of the candidates identified the correct types and numbers of bonds, and many calculated the enthalpy change of reaction correctly gaining full marks. Common mistakes included reversing the signs of bonds broken and bonds formed, and using incorrect types or numbers of bonds, and arithmetic errors.
(i) Less than half of the candidates answered the question correctly. Some were not specific in the definition of the standard enthalpy change of formation, while others had totally incorrect answers such as the formation of the compound from gaseous atoms.
(ii) The majority of candidates calculated the enthalpy change correctly. Some candidates made arithmetic errors.
More than half of the candidates referred to bond enthalpies being average values that lead to a less accurate calculated value of the enthalpy change.

