| Date | May 2011 | Marks available | 1 | Reference code | 11M.1.hl.TZ1.15 |
| Level | HL | Paper | 1 | Time zone | TZ1 |
| Command term | Deduce | Question number | 15 | Adapted from | N/A |
Question
When hydrogen peroxide decomposes, the temperature of the reaction mixture increases.
\[{\text{2}}{{\text{H}}_{\text{2}}}{{\text{O}}_{\text{2}}}{\text{(aq)}} \to {{\text{O}}_{\text{2}}}{\text{(g)}} + {\text{2}}{{\text{H}}_{\text{2}}}{\text{O(l)}}\]
What are the signs of \(\Delta H\), \(\Delta S\) and \(\Delta G\) for this reaction?
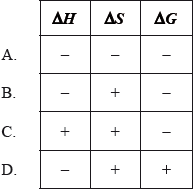
Markscheme
B
Examiners report
[N/A]
Syllabus sections
Show 71 related questions
- 17N.2.hl.TZ0.5c: The standard free energy change, ΔGθ, for the above reaction is –103 kJ mol–1 at 298...
- 17N.2.hl.TZ0.5b: Calculate the standard entropy change for this reaction using the following data.
- 17N.1.hl.TZ0.17: The combustion of glucose is exothermic and occurs according to the following...
- 17N.1.hl.TZ0.16: What is the standard enthalpy of formation, in kJ mol–1, of IF (g)? IF7 (g) + I2 (s) → IF5...
- 17M.2.hl.TZ2.9b.ii: The standard enthalpy change, ΔH θ, for the hydrogenation of propene is –124.4 kJ mol–1....
- 17M.2.hl.TZ2.9b.i: Hydrogenation of propene produces propane. Calculate the standard entropy change, ΔS θ, for...
- 17M.2.hl.TZ1.4c.iii: Use your answers to (c)(i) and (c)(ii), to determine the temperature, in °C, at which the...
- 17M.2.hl.TZ1.4c.ii: Calculate a value for \(\Delta {H^\theta }\) in kJ.
- 17M.2.hl.TZ1.4c.i: Calculate the standard entropy change, \(\Delta {S^\theta }\), of the reaction, in...
- 17M.2.hl.TZ1.3c.ii: Comment on the spontaneity of this reaction by calculating a value for \(\Delta {G^\theta...
- 17M.1.hl.TZ1.17: Which combination of ΔH θ and ΔS θ will result in a non-spontaneous reaction at all...
- 16N.2.hl.TZ0.1b: (i) Calculate ΔHθ, in kJ, for this similar reaction below using \(\Delta H_{\rm{f}}^\theta \)...
- 16M.2.hl.TZ0.2a: (i) Deduce the equilibrium constant expression, Kc, for this reaction. (ii) At exactly 600°C...
- 16M.1.hl.TZ0.19: What are the signs for the entropy...
- 15M.1.hl.TZ1.17: Which species are arranged in order of increasing entropy? A. ...
- 15M.1.hl.TZ1.18: Which combination of \(\Delta H\) and \(\Delta S\) values corresponds to a non-spontaneous...
- 15M.1.hl.TZ2.18: Which combinations of values will result in a spontaneous reaction? A. I and II...
- 15M.2.hl.TZ1.5f.ii: Deduce, giving a reason, the sign of the standard entropy change of the system for the...
- 15M.2.hl.TZ2.3b: Calculate the standard free energy change, \(\Delta {G^\Theta }\), in...
- 15M.2.hl.TZ2.3c: Using the values obtained in parts (a) and (b), calculate the standard entropy change,...
- 15M.2.hl.TZ2.3d: Determine the absolute entropy, \({S^\Theta }\), in...
- 14M.1.hl.TZ2.15: Which combination of enthalpy change and entropy change produces a non-spontaneous reaction...
- 14M.1.hl.TZ2.17: In which reaction will the entropy of the system increase significantly? A. ...
- 14M.1.hl.TZ1.17: Which reaction has the greatest increase in entropy? A. ...
- 14M.1.hl.TZ1.18: Which change must be negative when a reaction occurs spontaneously? A. \(\Delta H\) B....
- 14M.2.hl.TZ1.4c: Calculate the standard free energy change, \(\Delta {G^\Theta }\), in...
- 14M.2.hl.TZ1.4d: (i) Determine the standard entropy change of the reaction, \(\Delta {S^\Theta }\), at 298...
- 14N.1.hl.TZ0.16: Which processes have a negative value for \(\Delta {S^\Theta }\)? I. ...
- 14N.2.hl.TZ0.2d: Predict the sign of the entropy change of the reaction, \(\Delta S\), giving a reason.
- 13N.1.hl.TZ0.18: Which processes are predicted to have a positive entropy change, \(\Delta S\)? I. ...
- 13N.1.hl.TZ0.19: Which combination of \(\Delta H\) and \(\Delta S\) signs will always result in a spontaneous...
- 13N.2.hl.TZ0.5d: The entropy change, \(\Delta S\), for the decomposition of trinitramide has been estimated as...
- 13N.2.hl.TZ0.5e: Using \( + 700{\text{ J}}\,{{\text{K}}^{ - 1}}{\text{mo}}{{\text{l}}^{ - 1}}\) as the value...
- 13N.2.hl.TZ0.5f: Explain how changing the temperature will affect whether or not the decomposition of...
- 13M.1.hl.TZ1.17: Which process would be expected to have a \(\Delta {S^\Theta }\) value which is negative? A....
- 13M.1.hl.TZ1.18: When solid potassium chlorate, \({\text{KCl}}{{\text{O}}_{\text{3}}}\), dissolves in...
- 13M.2.hl.TZ1.8b.ii: Calculate the standard entropy change for this reaction, \(\Delta {S^\Theta }\), using Table...
- 13M.2.hl.TZ1.8b.iv: Predict, with a reason, the effect of an increase in temperature on the spontaneity of this...
- 13M.2.hl.TZ1.8b.iii: Calculate, stating units, the standard free energy change for this reaction,...
- 13M.1.hl.TZ2.17: Which reactions/processes have a positive entropy change, \(\Delta {S^\Theta }\)? I. ...
- 13M.2.hl.TZ2.5a.v: Determine the standard free energy change for the reaction, \(\Delta {G^\Theta }\), in...
- 13M.2.hl.TZ2.5a.vi: Deduce the temperature, in K, at which the reaction becomes spontaneous.
- 13M.2.hl.TZ2.5a.i: Predict and explain the sign of the entropy change, \(\Delta S\), for this reaction.
- 13M.2.hl.TZ2.5a.ii: Calculate the standard entropy change for the reaction, \(\Delta {S^\Theta }\), in...
- 12N.1.hl.TZ0.18: Consider the following...
- 10N.1.hl.TZ0.18: Which reaction has the largest increase in entropy? A. ...
- 10N.2.hl.TZ0.7c: (i) The enthalpy change of formation, \(\Delta H_{\text{f}}^\Theta \), of liquid...
- 09N.1.hl.TZ0.18: Which change leads to an increase in entropy? A. ...
- 09N.2.hl.TZ0.7b.ii: Predict, stating a reason, whether the sign of \({\Delta {S^\Theta }}\) for the above...
- 09M.1.hl.TZ1.16: Which reaction has the greatest increase in entropy? A. ...
- 09M.1.hl.TZ1.18: A reaction has a standard enthalpy change, \(\Delta {H^\Theta }\), of...
- 09M.1.hl.TZ1.17: The reaction between but-1-ene and water vapour produces...
- 09M.1.hl.TZ2.18: What is the standard entropy change, \(\Delta {S^\Theta }\), for the following...
- 09M.2.hl.TZ2.4b: The standard entropy for \({\text{C}}{{\text{H}}_{\text{3}}}{\text{OH(g)}}\) at 298 K is...
- 09M.2.hl.TZ2.4c: Calculate the standard change in free energy, at 298 K, for the reaction and deduce whether...
- 11M.2.hl.TZ1.1e: Using the enthalpy of combustion for methanol from Table 12 of the Data Booklet and the...
- 11M.1.hl.TZ1.16: Which reaction has the greatest increase in entropy? A. ...
- 11M.2.hl.TZ1.1d: Determine the \(\Delta {S^\Theta }\) for the combustion of...
- 11M.2.hl.TZ1.1f: Explain whether changing the temperature will alter the spontaneity of the reaction.
- 11M.1.hl.TZ2.19: \(\Delta {G^\Theta }\) calculations predict that a reaction is always spontaneous for which...
- 11M.2.hl.TZ2.3d: Some words used in chemistry can have a specific meaning which is different to their meaning...
- 11M.2.hl.TZ2.6b.iii: Calculate the standard entropy change for the hydrogenation of propene.
- 11M.2.hl.TZ2.6b.iv: Determine the value of \(\Delta {G^\Theta }\) for the hydrogenation of propene at 298 K.
- 11M.2.hl.TZ2.6b.v: At 298 K the hydrogenation of propene is a spontaneous process. Determine the temperature...
- 12M.1.hl.TZ2.17: During which process is there a decrease in the entropy of the system? A. ...
- 11N.1.hl.TZ0.18: Which factors will increase the entropy of this...
- 11N.1.hl.TZ0.21: The rate expression for the reaction between iodine and propanone with an acid catalyst is...
- 11N.2.hl.TZ0.5b.iii: Determine the standard free energy change at 298 K for the reaction. Deduce whether or not...
- 11N.2.hl.TZ0.5b.v: Estimate the temperature, in K, at which the standard change in free energy equals zero. You...
- 11N.2.hl.TZ0.5a: Deduce and explain the sign of the entropy change for the following...
- 11N.2.hl.TZ0.5b.iv: Determine the standard entropy change at 298 K for the reaction.

