| Date | May 2015 | Marks available | 1 | Reference code | 15M.2.hl.TZ2.3 |
| Level | HL | Paper | 2 | Time zone | TZ2 |
| Command term | Calculate | Question number | 3 | Adapted from | N/A |
Question
Carbon monoxide reacts with hydrogen to produce methanol.
\[{\text{CO(g)}} + {\text{2}}{{\text{H}}_{\text{2}}}{\text{(g)}} \to {\text{C}}{{\text{H}}_{\text{3}}}{\text{OH(l)}}\]
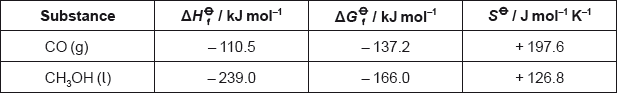
Calculate the standard enthalpy change, \(\Delta {H^\Theta }\), in \({\text{kJ}}\,{\text{mo}}{{\text{l}}^{ - {\text{1}}}}\), for the reaction.
Calculate the standard free energy change, \(\Delta {G^\Theta }\), in \({\text{kJ}}\,{\text{mo}}{{\text{l}}^{ - {\text{1}}}}\), for the reaction
\({\text{(}}\Delta G_{\text{f}}^\Theta {\text{(}}{{\text{H}}_{\text{2}}}{\text{)}} = {\text{0 kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}{\text{)}}\).
Using the values obtained in parts (a) and (b), calculate the standard entropy change, \(\Delta {S^\Theta }\), in \({\text{J mo}}{{\text{l}}^{ - 1}}{{\text{K}}^{ - 1}}\), for the reaction at 298 K.
Determine the absolute entropy, \({S^\Theta }\), in \({\text{J}}\,{\text{mo}}{{\text{l}}^{ - 1}}{{\text{K}}^{ - 1}}\), for \({{\text{H}}_{\text{2}}}{\text{(g)}}\) at 298 K.
Markscheme
\(( - 239.0 - [ - 110.5] = ) - 128.5{\text{ (kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}{\text{)}}\);
\(( - 166.0 - [ - 137.2] = ) - 28.8{\text{ (kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}{\text{)}}\);
\(\left( {\Delta {G^\Theta } = - 28.8 = - 128.5 - \left[ {\frac{{298 \times \Delta {S^\Theta }}}{{1000}}} \right]} \right)\)
\((\Delta {S^\Theta } = ) - 335{\text{ (J}}\,{\text{mo}}{{\text{l}}^{ - 1}}{{\text{K}}^{ - 1}})\);
\(\Delta {S^\Theta } = \sum {S_{products}^\Theta - \sum {S_{reactants}^\Theta } } / - 335 = 126.8 - 197.6 - 2S_{{{\text{H}}_2}}^\Theta \);
\(S_{{{\text{H}}_2}}^\Theta = ( + )132{\text{ (J}}\,{\text{mo}}{{\text{l}}^{ - 1}}{{\text{K}}^{ - 1}})\);
Award [2] for correct final answer.
Award [1 max] for \(S_{{{\text{H}}_2}}^\Theta = ( + )264{\text{ (J}}\,{\text{mo}}{{\text{l}}^{ - 1}}{{\text{K}}^{ - 1}})\).
Examiners report
Most candidates were able to calculate the enthalpy, free energy and entropy changes, although a significant number gave the incorrect units for the latter. The use of the Gibbs free energy equation requires consistency of units since \(\Delta H{^\circ _{\text{f}}}\) and \(\Delta G{^\circ _{\text{f}}}\) were given in kJ whereas \(S^\circ \) was given in J. This needs reinforcing in class as it tends to be a common error from session to session. The calculation of the absolute entropy of hydrogen proved to be more problematic, with many not taking into account that there are two moles of hydrogen in the reaction.
Most candidates were able to calculate the enthalpy, free energy and entropy changes, although a significant number gave the incorrect units for the latter. The use of the Gibbs free energy equation requires consistency of units since \(\Delta H{^\circ _{\text{f}}}\) and \(\Delta G{^\circ _{\text{f}}}\) were given in kJ whereas \(S^\circ \) was given in J. This needs reinforcing in class as it tends to be a common error from session to session. The calculation of the absolute entropy of hydrogen proved to be more problematic, with many not taking into account that there are two moles of hydrogen in the reaction.
Most candidates were able to calculate the enthalpy, free energy and entropy changes, although a significant number gave the incorrect units for the latter. The use of the Gibbs free energy equation requires consistency of units since \(\Delta H{^\circ _{\text{f}}}\) and \(\Delta G{^\circ _{\text{f}}}\) were given in kJ whereas \(S^\circ \) was given in J. This needs reinforcing in class as it tends to be a common error from session to session. The calculation of the absolute entropy of hydrogen proved to be more problematic, with many not taking into account that there are two moles of hydrogen in the reaction.
Most candidates were able to calculate the enthalpy, free energy and entropy changes, although a significant number gave the incorrect units for the latter. The use of the Gibbs free energy equation requires consistency of units since \(\Delta H{^\circ _{\text{f}}}\) and \(\Delta G{^\circ _{\text{f}}}\) were given in kJ whereas \(S^\circ \) was given in J. This needs reinforcing in class as it tends to be a common error from session to session. The calculation of the absolute entropy of hydrogen proved to be more problematic, with many not taking into account that there are two moles of hydrogen in the reaction.

