| Date | November 2014 | Marks available | 2 | Reference code | 14N.3.SL.TZ0.5 |
| Level | Standard level | Paper | Paper 3 | Time zone | Time zone 0 |
| Command term | Show that | Question number | 5 | Adapted from | N/A |
Question
This question is about the wave nature of matter.
In 1927 Davisson and Germer tested the de Broglie hypothesis. They directed a beam of electrons onto a nickel crystal as shown in the diagram. The experiment was carried out in a vacuum.
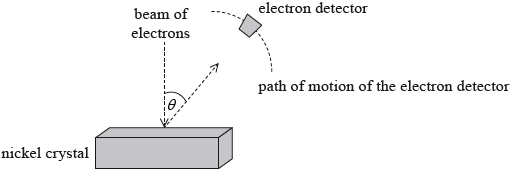
Describe wave-particle duality in relation to the de Broglie hypothesis.
The electrons were accelerated through a potential difference of 54 V. Show that the associated de Broglie wavelength for the electrons is about \(2 \times {10^{ - 10}}{\text{ m}}\).
The electron detector recorded a large number of electrons at a particular scattering angle \(\theta \). Explain why a maximum in the number of scattered electrons is observed at a particular angle.
Markscheme
a particle with momentum has a wavelength ;
where \(\lambda = \frac{h}{p}\) and wavelength is \(\lambda \), momentum is \(p\) and Planck’s constant is \(h\);
\(p = \sqrt {2{m_{\text{e}}} \times eV} = \sqrt {2 \times 9.11 \times {{10}^{ - 31}} \times 1.60 \times {{10}^{ - 19}} \times 54} {\text{ }}( = 3.97 \times {10^{ - 24}}{\text{ kg}}\,{\text{m}}\,{{\text{s}}^{ - 1}})\);
\(\lambda = \left( {\frac{h}{{\sqrt {2{m_{\text{e}}} \times eV} }} = \frac{{6.63 \times {{10}^{ - 34}}}}{{3.97 \times {{10}^{ - 24}}}} = } \right){\text{ }}1.7 \times {10^{ - 10}}{\text{ m}}\); }
(must see 2+ significant figures to award this mark)
electrons scatter off the periodic structure of the nickel lattice;
separation of ions/atoms is such that diffraction occurs (leading to a maximum in the intensity of scattered electrons);
Examiners report
(a) was usually well answered.
(b)(i) was either well answered or incorrect.
(b)(ii) was very poorly answered.
Syllabus sections
- 17N.1.HL.TZ0.40: A photon interacts with a nearby nucleus to produce an electron. What is the name of this...
- 17N.1.HL.TZ0.39: Monochromatic electromagnetic radiation is incident on a metal surface. The kinetic energy of...
- 17N.1.HL.TZ0.23: Samples of different radioactive nuclides have equal numbers of nuclei. Which graph shows...
- 17M.2.HL.TZ2.7c.ii: State and explain the effect on the maximum photoelectric current as a result of increasing...
- 17M.2.HL.TZ2.7c.i: Describe the change in the number of photons per second incident on the surface of the...
- 17M.2.HL.TZ2.7b: Radiation of photon energy 5.2 x 10–19 J is now incident on the photocell. Calculate...
- 17M.2.HL.TZ2.7a.ii: Electrons emitted from the surface of the photocell have almost no kinetic energy. Explain...
- 17M.2.HL.TZ2.7a.i: Calculate the wavelength of the light.
- 17M.2.HL.TZ1.9c: The experiment is repeated with a metal surface of cadmium, which has a greater...
- 17M.2.HL.TZ1.9b.iii: Calculate the work function of barium in eV.
- 17M.2.HL.TZ1.9b.ii: State what is meant by the work function of a metal.
- 17M.2.HL.TZ1.9b.i: Determine a value for Planck’s constant.
- 17M.2.HL.TZ1.9a: Explain how each observation provides support for the particle theory but not the wave theory...
- 17M.1.HL.TZ2.39: A neutron of mass m is confined within a nucleus of diameter d. Ignoring numerical...
- 17M.1.HL.TZ2.38: In the Bohr model for hydrogen an electron in the ground state has orbit radius r and speed...
- 17M.1.HL.TZ1.39: A photon of energy E and wavelength λ is scattered from an electron initially at rest. What...
- 17M.1.HL.TZ1.38: What can be used to calculate the probability of finding an electron in a particular region...
- 16M.2.HL.TZ0.11c: An alpha particle is confined within a nucleus of gold. Using the uncertainty principle,...
- 16N.2.HL.TZ0.11b: The graph shows the variation of photoelectric current I with potential difference V between...
- 16N.2.HL.TZ0.11a: A current is observed on the ammeter when violet light illuminates C. With V held constant...
- 16N.1.HL.TZ0.38: An electron of mass m has an uncertainty in its position r. What is the uncertainty in the...
- 16N.1.HL.TZ0.37: Pair production by a photon occurs in the presence of a nucleus. For this process, which of...
- 16M.1.HL.TZ0.38: Different...
- 16M.1.HL.TZ0.36: The graphs show the...
- 16M.1.HL.TZ0.35: Which of the following...
- 15M.1.HL.TZ1.29: Photoelectrons are emitted at a certain rate when monochromatic light is incident on a metal...
- 15M.1.HL.TZ1.30: Which phenomenon provides evidence for the wave nature of an electron? A. Line spectra of...
- 15M.1.HL.TZ2.28: Red light incident on a metal surface produces photoelectrons. The potential V of the...
- 15M.2.HL.TZ1.7c: Explain how the pattern demonstrates that electrons have wave properties.
- 15M.2.HL.TZ1.7d: Electrons are accelerated to a speed of 3.6×107 ms−1 by the electric field. (i) Calculate...
- 15M.2.HL.TZ1.7e: State what can be deduced about an electron from the amplitude of its associated wavefunction.
- 15M.2.HL.TZ1.7f: An electron reaching the central bright spot on the fluorescent screen has a small...
- 15M.2.HL.TZ2.9d: An electron is confined in a length of 2.0 \( \times \) 10–10 m. (i) Determine the...
- 15M.2.HL.TZ2.9c: State what is meant by the wavefunction of an electron.
- 15M.3.SL.TZ1.5b: Determine the maximum wavelength of the photons that can cause photoemission.
- 15M.3.SL.TZ1.5c: Calculate the momentum of an electron that has the same de Broglie wavelength as the...
- 15M.3.SL.TZ2.5a: (i) Calculate, in eV, the maximum kinetic energy of the emitted electrons. (ii) The number...
- 15M.3.SL.TZ2.5b: The wavelength of the light incident on the sodium surface is decreased without changing its...
- 14M.1.HL.TZ1.33: In the “electron in a box” model, an electron is confined to move along a line of length L....
- 14M.1.HL.TZ2.28: Light that is shone onto a metal surface may result in the emission of electrons from the...
- 14M.1.HL.TZ2.29: An electron X is accelerated from rest through a potential difference V. Another electron Y...
- 14M.1.HL.TZ2.31: If there is no uncertainty in the value of the de Broglie wavelength of a particle then this...
- 14M.2.HL.TZ1.9f: Light is incident on a metal surface A. A potential difference is applied between A and an...
- 14M.2.HL.TZ1.9g: A photon of energy 6.6×10–19J is incident upon a clean sodium surface. The work function of...
- 15N.1.HL.TZ0.28: When electromagnetic radiation falls on a photocell, electrons of mass \({m_{\text{e}}}\) are...
- 15N.2.HL.TZ0.5b.ii: The intensity of the light is \({\text{5.1 }}\mu {\text{W}}\,{{\text{m}}^{ - 2}}\). Determine...
- 15N.1.HL.TZ0.30: A particle has a de Broglie wavelength \(\lambda \) and kinetic energy \(E\). What is the...
- 15N.2.HL.TZ0.5b.i: Calculate, in eV, the maximum kinetic energy of the photoelectrons emitted.
- 15N.2.HL.TZ0.5a: Outline why the wave model of light cannot account for the photoelectric effect.
- 14M.3.SL.TZ1.4a: Describe the de Broglie hypothesis.
- 14M.3.SL.TZ1.4c: The momentum of the electron is known precisely. Deduce that all the information on its...
- 14M.3.SL.TZ1.4d: With reference to Schrödinger’s model, state the meaning of the amplitude of the wavefunction...
- 15N.3.SL.TZ0.5a: Outline how the Einstein model is used to explain the photoelectric effect.
- 15N.3.SL.TZ0.5b: State why, although the incident light is monochromatic, the energies of the emitted...
- 15N.3.SL.TZ0.5c: Explain why no electrons are emitted if the frequency of the incident light is less than a...
- 15N.3.SL.TZ0.5d: For monochromatic light of wavelength 620 nm a stopping potential of 1.75 V is required....
- 15N.3.SL.TZ0.6a.i: On the diagram, label using arrows all the possible transitions that might occur as the...
- 15N.3.SL.TZ0.6a.ii: State the energy in eV of the maximum wavelength photon emitted as the hydrogen atom returns...
- 15N.3.SL.TZ0.6b.i: 10.2 eV.
- 15N.3.SL.TZ0.6b.ii: 9.0 eV.
- 14N.1.HL.TZ0.29: Which of the following is correct for the de Broglie wavelength λ of a particle when the...
- 14N.1.HL.TZ0.31: According to the Heisenberg uncertainty principle, conjugate quantities are pairs of...
- 14N.1.HL.TZ0.32: Three phenomena associated with nuclear and quantum physics are I. Einstein photoelectric...
- 14N.2.HL.TZ0.9d: Explain why photoelectrons are not emitted from the metal surface unless the frequency of...
- 14N.2.HL.TZ0.9e.i: identify the minimum value of the frequency \({f_0}\) for photoelectrons to be emitted.
- 14N.2.HL.TZ0.9e.ii: determine the Planck constant.
- 14N.2.HL.TZ0.9e.iii: calculate the work function, in eV, for the metal surface.
- 14N.2.HL.TZ0.9f: The student repeats the experiment with a different metal surface that has a smaller...
- 14N.3.SL.TZ0.5a: Describe wave-particle duality in relation to the de Broglie hypothesis.
- 14N.3.SL.TZ0.5b.ii: The electron detector recorded a large number of electrons at a particular scattering angle...
- 14M.2.HL.TZ2.8c: State what is meant by the photoelectric effect.
- 14M.2.HL.TZ2.8d: (i) Suggest why the work function for caesium is smaller than that of mercury. (ii) ...
- 14M.2.HL.TZ2.8f: An exact determination of the location of the electron in a hydrogen atom is not possible....
- 14M.3.SL.TZ2.4a: Describe what is meant by the de Broglie hypothesis.
- 14M.3.SL.TZ2.4b: (i) Calculate the kinetic energy of the particle. (ii) Determine the de Broglie...
- 11N.1.HL.TZ0.30: The probability of finding an electron at a particular position in a hydrogen atom is...
- 11N.1.HL.TZ0.28: A positively charged particle of charge q and mass m is accelerated from rest through a...
- 11N.1.HL.TZ0.29: Light is shone onto the surface of a metal and photoelectrons are emitted. Which of the...
- 12N.1.HL.TZ0.33: According to the Heisenberg uncertainty principle the quantity paired with momentum is A....
- 12N.1.HL.TZ0.34: Photons are incident on a metal surface. Electrons are emitted from the surface. What single...
- 13N.1.HL.TZ0.28: When the cathode of a photoelectric cell is illuminated with red light, a photoelectric...
- 13N.1.HL.TZ0.30: In the Heisenberg uncertainty principle, conjugate quantities are pairs of quantities that...
- 13M.1.HL.TZ1.29: An electron accelerated from rest through a potential difference V has de Broglie wavelength...
- 12M.1.HL.TZ2.29: An electron of mass me and a proton of mass mp are moving with the same kinetic energy at...
- 12M.1.HL.TZ2.40: Photoelectrons are emitted from the surface of a metal when light of frequency ƒ is incident...
- 13M.2.HL.TZ1.8a: State what is meant by work function.
- 13M.2.HL.TZ1.8d: In an experiment, light at a particular frequency is incident on a surface and electrons are...
- 13M.2.HL.TZ1.8b: The diagram shows part of an experimental arrangement used to investigate the photoelectric...
- 13M.3.SL.TZ1.5b: Outline how the de Broglie hypothesis explains the existence of a discrete set...
- 13M.3.SL.TZ1.5c: The diagram below shows the shape of two allowed wavefunctions ѱA and ѱB for an electron...
- 11M.1.HL.TZ2.27: Monoc...
- 11M.1.HL.TZ2.30: ...
- 12M.1.HL.TZ1.28: Light of a particular wavelength and intensity does not cause photoelectric emission from a...
- 12M.1.HL.TZ1.29: Alpha particles of charge +2e and mass m are accelerated from rest through a potential...
- 11M.2.HL.TZ2.12a: Explain with reference to the Einstein model, which graph, A or B,...
- 11M.2.HL.TZ2.12b: The frequency of the light that produces graph A is 8.8×1014Hz....
- 11M.2.HL.TZ2.12c: The frequency of the incident light is increased but the...
- 11M.2.HL.TZ2.12d: The electrons emitted from the photo-cathode have...
- 12M.2.HL.TZ1.15c: Consider an electron confined in a one-dimensional “box” of length L. The de Broglie waves...
- 12M.2.HL.TZ1.15d: An electron is confined in a “box” of length L=1.0×10–10m in the n=1 energy level. Its...
- 12M.3.SL.TZ1.5a: When red light is incident on the metallic surface M the microammeter registers a current....
- 12M.3.SL.TZ1.5b: The graph shows the variation with voltage V of the current I in the circuit. The work...
- 12M.3.SL.TZ1.12d: The total energy of the particle represented by the dotted line is 1.2 GeV more than what is...
- 11M.3.SL.TZ2.4c: Explain why a precise knowledge of the de Broglie wavelength of the proton implies that its...
- 11M.3.SL.TZ2.3a: State what is meant by the photoelectric effect.
- 11M.3.SL.TZ2.3b: Light of frequency 8.7×1014Hz is incident on the surface of a metal in a photocell. The...
- 11M.3.SL.TZ2.4b: Determine the de Broglie wavelength of a proton that has been accelerated from rest through a...
- 12M.3.SL.TZ2.5a: Describe the concept of a photon.
- 11M.3.SL.TZ2.4a: State the de Broglie hypothesis.
- 12M.3.SL.TZ2.5b: In the photoelectric effect there exists a threshold frequency below which no emission...
- 11N.3.SL.TZ0.4b: Light of frequency f is shone onto the tungsten electrode in (a). The potential Vs for which...
- 11N.3.SL.TZ0.4a: The diagram shows the set up of an experiment designed to verify the Einstein model of the...
- 11N.3.SL.TZ0.4c: The work function of tungsten is 4.5eV. Show that the de Broglie wavelength of an electron...
- 12N.2.HL.TZ0.10b: The wavelength of the incident light in (a) is 420 nm and the work function of the metal is...
- 12N.2.HL.TZ0.10a: Monochromatic light is incident on a metal surface and electrons are emitted instantaneously...
- 12M.3.SL.TZ2.5c: Light of wavelength 420 nm is incident on a clean metal surface. The work function of the...
- 11M.1.HL.TZ1.28: The diagram below shows a circuit involving a photoelectric cell. When UV light is shone onto...
- 11M.1.HL.TZ1.31: A proton is confined within a nucleus. What is the order of magnitude of the uncertainty in...
- 13N.3.SL.TZ0.4a: Monochromatic light of different frequencies is incident on a metal surface placed in a...
- 13N.3.SL.TZ0.4b: The graph shows how the maximum kinetic energy EK of the ejected electrons in (a) varies with...
- 13N.3.SL.TZ0.4c: Show that electrons of energy 0.50 eV have a de Broglie wavelength of about 1.7×10–9m.
- 11M.2.HL.TZ1.10a: State what is meant by a wavefunction.
- 11M.2.HL.TZ1.10c: Calculate the momentum of the electron.
- 11M.2.HL.TZ1.10e: The electron stays in the first excited state of hydrogen for a time of...
- 11M.1.HL.TZ1.29: Electrons are accelerated from rest through a potential difference V. Their de Broglie...
- 11M.2.HL.TZ1.10b: State the position near which this electron is most likely to be found.
- 11M.2.HL.TZ1.10d: The energy, in joules, of the electron in a hydrogen atom, is given by...
- 11M.3.SL.TZ1.4: This question is about the photoelectric effect. In an experiment to investigate the...
- 09M.1.HL.TZ1.31: In the Schrödinger model of the hydrogen atom, the probability of finding an electron in a...
- 09M.1.HL.TZ1.26: Ultra-violet light is shone on a zinc surface and photoelectrons are emitted. The sketch...
- 10M.1.HL.TZ1.28: Light of frequency \(f\) is incident on a metal surface. The work function of the metal is...
- 10M.1.HL.TZ1.30: Which of the following is an assumption of the Schrödinger model of the hydrogen atom? A. ...
- 10M.1.HL.TZ1.29: An electron is accelerated from rest through a potential difference \(V\). Which of the...
- 09N.1.HL.TZ0.32: A particle is accelerated from rest through a potential difference \(V\). Which of the...
- 10N.1.HL.TZ0.31: In the photoelectric effect, the following observations may be made. I. The kinetic...
- 09N.1.HL.TZ0.33: Which of the following is a correct statement associated with the photoelectric effect? A. ...
- 09N.1.HL.TZ0.31: The square of the amplitude of the electron wave function in an hydrogen atom is a measure of...
- 10N.1.HL.TZ0.32: A proton and an alpha particle have the same de Broglie wavelength. Which of the following...
- 10N.3.SL.TZ0.B1a: (i) Explain, with reference to the Einstein model of the photoelectric effect, the...
- 10N.3.SL.TZ0.B1b: (i) Show that the maximum kinetic energy of the emitted electrons is...

