| Date | November 2015 | Marks available | 1 | Reference code | 15N.3.SL.TZ0.6 |
| Level | Standard level | Paper | Paper 3 | Time zone | Time zone 0 |
| Command term | Describe | Question number | 6 | Adapted from | N/A |
Question
This question is about energy level transitions.
Some of the electron energy levels for a hydrogen atom are shown.
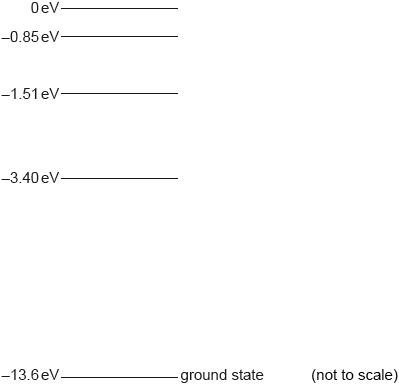
A hydrogen atom is excited to the \( - 1.51{\text{ eV}}\) level.
Monochromatic radiation is incident on gaseous hydrogen. All the hydrogen atoms are in the ground state. Describe what could happen to the radiation and to the hydrogen atoms if the incident photon energy is equal to
On the diagram, label using arrows all the possible transitions that might occur as the hydrogen atom returns to the ground state.
State the energy in eV of the maximum wavelength photon emitted as the hydrogen atom returns to the ground state.
10.2 eV.
9.0 eV.
Markscheme
only the three correct arrows on diagram;
(–1.51 to –3.40, –1.15 to –13.6 and –3.40 to –13.6)
1.89 eV; (allow ECF from diagram)
photon is absorbed;
electron (in a hydrogen atom) raised to higher/–3.40 eV/excited state;
no absorption / photon pass through;
Examiners report
The hydrogen atom energy diagram was generally well-answered. The energy of the maximum wavelength was usually confused with the maximum frequency. The description of the photons of different energies were usually answered incompletely, not referring to both the radiation and the hydrogen atoms. A number of candidates referred to hydrogen atoms jumping energy levels, rather than electrons.
The hydrogen atom energy diagram was generally well-answered. The energy of the maximum wavelength was usually confused with the maximum frequency. The description of the photons of different energies were usually answered incompletely, not referring to both the radiation and the hydrogen atoms. A number of candidates referred to hydrogen atoms jumping energy levels, rather than electrons.
The hydrogen atom energy diagram was generally well-answered. The energy of the maximum wavelength was usually confused with the maximum frequency. The description of the photons of different energies were usually answered incompletely, not referring to both the radiation and the hydrogen atoms. A number of candidates referred to hydrogen atoms jumping energy levels, rather than electrons.
The hydrogen atom energy diagram was generally well-answered. The energy of the maximum wavelength was usually confused with the maximum frequency. The description of the photons of different energies were usually answered incompletely, not referring to both the radiation and the hydrogen atoms. A number of candidates referred to hydrogen atoms jumping energy levels, rather than electrons.

