| Date | May 2009 | Marks available | 3 | Reference code | 09M.3.hl.TZ2.F3 |
| Level | HL | Paper | 3 | Time zone | TZ2 |
| Command term | Explain | Question number | F3 | Adapted from | N/A |
Question
Lycopene, whose structure is shown below, is a carotenoid and is responsible for the red colour in tomatoes. When bromine is slowly added to some tomato juice, the colour of the juice gradually changes from red to yellow. Explain this colour change in terms of changes in bonding in lycopene.
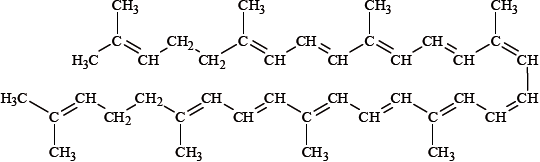
Markscheme
\({\text{B}}{{\text{r}}_{\text{2}}}\) reacts with the double bonds / amount of double bonds in the conjugated system decreases;
absorbed energy shifts to violet/higher energy in the visible region is absorbed;
resulting in complementary yellow colour;
Examiners report
Most candidates were unable to relate the addition of bromine to bond saturation, the shift of energy absorbance to violet/higher energy in the visible region and the transmittance of the complementary yellow light.

