| Date | May 2010 | Marks available | 2 | Reference code | 10M.3.sl.TZ1.F2 |
| Level | SL | Paper | 3 | Time zone | TZ1 |
| Command term | Explain | Question number | F2 | Adapted from | N/A |
Question
The wavelength of visible light lies between 400 and 750 nm. The absorption spectrum of a particular anthocyanin is shown below.
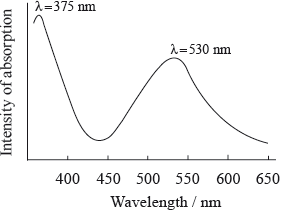
Explain why pigments such as anthocyanins are coloured.
(i) Explain what effect, if any, the absorption at 375 nm will have on the colour of the anthocyanin.
(ii) Explain what effect, if any, the absorption at 530 nm will have on the colour of the anthocyanin.
List two factors which could alter the precise colour of a particular anthocyanin.
Markscheme
pigments absorb visible light;
and scatter/reflect/transmit the remaining light;
(i) no effect as it lies outside the visible region/is in the UV / OWTTE;
(ii) the colour will be the complementary colour to the colour absorbing at 530 nm / it will be red as 530 nm is blue-green / OWTTE;
oxidation;
temperature;
pH/acidity/basicity;
presence of metal ions;
Examiners report
Many candidates failed to mention that visible light is absorbed by coloured pigments and that the complimentary colour is seen.
Part (b) was answered correctly by most candidates, although some neglected to refer to their Data Booklet to identify the regions of the spectrum.
The majority of candidates could list factors which alter the precise colour of a particular anthocyanin in part (c).

