| Date | May 2015 | Marks available | 1 | Reference code | 15M.3.hl.TZ2.28 |
| Level | HL | Paper | 3 | Time zone | TZ2 |
| Command term | Identify | Question number | 28 | Adapted from | N/A |
Question
The colour of olive oil is due to pigments such as chlorophyll, pheophytin and carotenoids.
The absorption spectrum of one form of pheophytin is shown below.
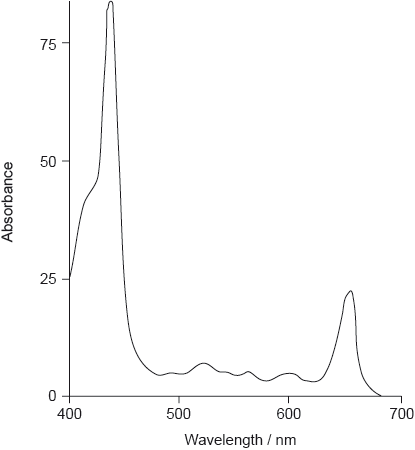
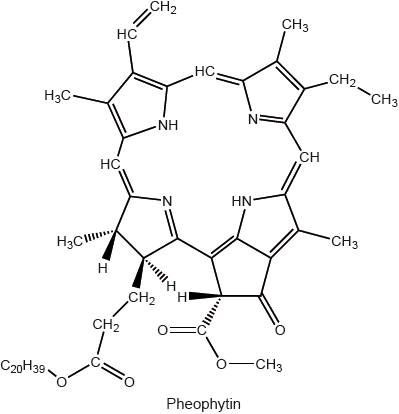
State the structural feature of a pheophytin molecule which allows it to absorb visible light.
Carotenoids may lose their colour and develop off odours when they are oxidized.
Identify, using table 22 of the data booklet, the structural feature that makes carotenoids susceptible to oxidation.
List two factors which increase the rate of oxidation of carotenoids.
Deduce, giving a reason, whether carotenoids are water-soluble or fat-soluble.
Markscheme
extensive conjugation/delocalization / extended system of conjugated double bonds/delocalized electrons;
Accept “contains porphyrin/porphin ring”.
multiple/conjugated C=C/carbon to carbon double bonds;
light;
higher/increased temperatures;
metals / transition metal ions;
hydroperoxides / peroxides;
Accept acidity/low pH.
fat-soluble and many non-polar groups/long non-polar chain;
Accept fat-soluble and absence of hydroxyl/OH/polar/H-bonding groups.
Examiners report
Though appreciation of the issue of complementary colours was widespread, hardly any candidates identified both absorption bands in the visible region. In the next few parts of the question many students lost marks by failing to answer fully, not referring to the extensive conjugation or the large number of carbon-carbon double bonds, as well as referring to temperature, rather than increased temperature, as a factor in the rate of oxidation. Lack of polarity was widely given as a reason for the fat solubility of carotenoids, though lack of groups able to form hydrogen bonds might have been more accurate (many quite polar molecules, such as \({\text{CHC}}{{\text{l}}_{\text{3}}}\), are insoluble in water). The features that lead to phospholipids acting as emulsifiers were often known, but again students tended to lose marks because their answers did not contain enough detail of their interaction between the phases present.
Though appreciation of the issue of complementary colours was widespread, hardly any candidates identified both absorption bands in the visible region. In the next few parts of the question many students lost marks by failing to answer fully, not referring to the extensive conjugation or the large number of carbon-carbon double bonds, as well as referring to temperature, rather than increased temperature, as a factor in the rate of oxidation. Lack of polarity was widely given as a reason for the fat solubility of carotenoids, though lack of groups able to form hydrogen bonds might have been more accurate (many quite polar molecules, such as \({\text{CHC}}{{\text{l}}_{\text{3}}}\), are insoluble in water). The features that lead to phospholipids acting as emulsifiers were often known, but again students tended to lose marks because their answers did not contain enough detail of their interaction between the phases present.
Though appreciation of the issue of complementary colours was widespread, hardly any candidates identified both absorption bands in the visible region. In the next few parts of the question many students lost marks by failing to answer fully, not referring to the extensive conjugation or the large number of carbon-carbon double bonds, as well as referring to temperature, rather than increased temperature, as a factor in the rate of oxidation. Lack of polarity was widely given as a reason for the fat solubility of carotenoids, though lack of groups able to form hydrogen bonds might have been more accurate (many quite polar molecules, such as \({\text{CHC}}{{\text{l}}_{\text{3}}}\), are insoluble in water). The features that lead to phospholipids acting as emulsifiers were often known, but again students tended to lose marks because their answers did not contain enough detail of their interaction between the phases present.
Though appreciation of the issue of complementary colours was widespread, hardly any candidates identified both absorption bands in the visible region. In the next few parts of the question many students lost marks by failing to answer fully, not referring to the extensive conjugation or the large number of carbon-carbon double bonds, as well as referring to temperature, rather than increased temperature, as a factor in the rate of oxidation. Lack of polarity was widely given as a reason for the fat solubility of carotenoids, though lack of groups able to form hydrogen bonds might have been more accurate (many quite polar molecules, such as \({\text{CHC}}{{\text{l}}_{\text{3}}}\), are insoluble in water). The features that lead to phospholipids acting as emulsifiers were often known, but again students tended to lose marks because their answers did not contain enough detail of their interaction between the phases present.

