| Date | November 2013 | Marks available | 3 | Reference code | 13N.3.hl.TZ0.5 |
| Level | HL | Paper | 3 | Time zone | TZ0 |
| Command term | Explain | Question number | 5 | Adapted from | N/A |
Question
A natural pigment found in cranberries can exist in two forms.
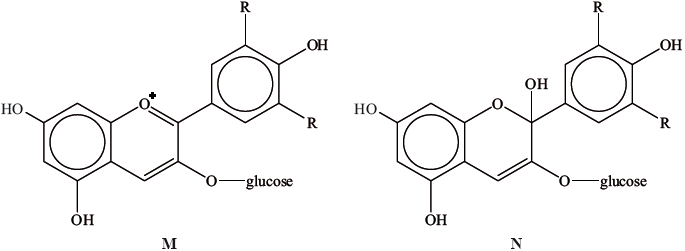
Explain, with reference to hybridization, which form is more likely to be coloured.
Markscheme
M as it has more delocalized pi electrons/extensive delocalized pi-bonding system/more conjugated;
M has many (linked) \({\text{s}}{{\text{p}}^{\text{2}}}\) carbon atoms / presence of \({\text{s}}{{\text{p}}^{\text{3}}}\) hybridized carbon in N limits delocalization/conjugation;
M absorbs light of longer wavelengths/shorter frequencies in the visible region;
Examiners report
Option A proved to be very popular. Most candidates were able to state spin as the property of protons that allows them to be detected by MRI but stated molecular spin. The advantage of MRI over X-ray of being able to detect soft tissue was well answered although some did not read the question carefully and stated the reduced health risk which was already mentioned in the question. Part 2 was generally well done by many candidates but with the following concerns: most arrived at the correct molecular mass, but then omitted positive sign on the molecular ion; a significant number drew the correct formula but with some fanciful formulae. Explaining the splitting pattern for the quartet caused the biggest challenge with very few scoring full marks; the H was incorrectly assigned to the OH (with results in no splitting due to rapid proton exchange) rather than the one attached to the C atom and the relative heights of the peaks (1:3:3:1) was not identified by almost all candidates.
Identification of infrared ranges was generally done correctly, but occasionally the wrong value was given in the similarities. The suggestion as to why HPLC is used for the detection of drug in urine sample was not done well with few scoring full marks; the understanding that the substance is non-volatile or decomposes at high temperatures was rarely identified. Also, identification of features that allow molecules to absorb UV radiation was not answered well. The question on atomic Absorption spectroscopy was answered with mixed results – some failed to specify that it must be an aluminium lamp and the idea of absorption of radiation (at Z) was missed by many. Most graphs were correctly drawn; however, some did not connect the line to the origin which was the first point in the data table; others blundered at reading off the graph. A significant number of candidates were able to use arguments related to delocalization and absorption in the visible range. However, hybridization was not often identified or used usually correctly.

