| Date | May 2009 | Marks available | 1 | Reference code | 09M.1.sl.TZ1.7 |
| Level | SL | Paper | 1 | Time zone | TZ1 |
| Command term | Determine | Question number | 7 | Adapted from | N/A |
Question
How many protons, neutrons and electrons are present in each atom of \(^{{\text{31}}}{\text{P}}\)?
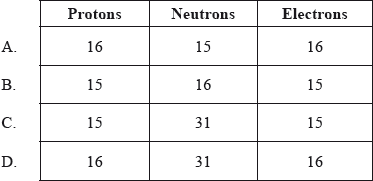
Markscheme
B
Examiners report
[N/A]
Syllabus sections
Show 70 related questions
- 17N.1.sl.TZ0.5: What is the number of protons and the number of neutrons in 131I?
- 17N.2.hl.TZ0.2c: A sample of magnesium has the following isotopic composition. Calculate the relative...
- 17M.2.sl.TZ2.1b: Naturally occurring silver is composed of two stable isotopes, 107Ag and 109Ag. The relative...
- 17M.1.sl.TZ2.5: What does \({}_{12}^{24}M{g^{2 + }}\) represent? A. An ion with 12 protons and 24...
- 17M.2.sl.TZ1.2c: State the number of protons, neutrons and electrons in the \({}_{22}^{48}{\text{Ti}}\) atom.
- 17M.2.sl.TZ1.2b: Titanium exists as several isotopes. The mass spectrum of a sample of titanium gave the...
- 17M.1.sl.TZ1.5: In which set do all the species contain more electrons than neutrons? A. 14N, 16O,...
- 16N.2.sl.TZ0.4b: Mass spectroscopic analysis of a sample of magnesium gave the following results: Calculate...
- 16N.2.sl.TZ0.4a: State the nuclear symbol notation, \({}_Z^AX\), for magnesium-26.
- 16N.1.hl.TZ0.5: Which representation would be correct for a species, Z, which has 31 protons, 40 neutrons and...
- 16M.2.sl.TZ0.4c: A sample of compound A was prepared in which the 12C in the CH2 group was replaced by...
- 16M.1.sl.TZ0.5: Which is correct for the chromium isotope...
- 15M.2.hl.TZ1.4b: State one difference in the physical properties of the isotopes \(^{{\text{63}}}{\text{Cu}}\)...
- 15M.1.sl.TZ1.5: Which statement about the isotopes of nitrogen is correct?
- 15M.1.sl.TZ2.5: Which statement is correct for the ion \(_4^9{\text{B}}{{\text{e}}^{2 + }}\)? A. The ion...
- 15M.1.sl.TZ2.6: Which ion will be deflected most in a mass spectrometer? A. \(^{16}{{\text{O}}^ +...
- 15M.2.sl.TZ1.4a: State the relative mass and charge of the subatomic particles of an atom.
- 15M.2.sl.TZ1.4b: (i) Calculate the number of neutrons and electrons in one atom of...
- 15M.2.sl.TZ2.2a: Calculate the number of protons, neutrons and electrons in the...
- 15M.2.sl.TZ2.2d: The sample contained the three isotopes \(^{{\text{24}}}{\text{Mg}}\),...
- 14M.2.hl.TZ2.2a: The relative atomic mass of copper is 63.55. Calculate the percentage of...
- 14M.1.sl.TZ2.6: Which species would be deflected most in a mass spectrometer? A. ...
- 14M.1.sl.TZ2.5: What does \(_{{\text{24}}}^{{\text{52}}}{\text{X}}\) represent? A. An isotope of Te with...
- 14M.2.sl.TZ2.2a: State the number of protons, neutrons and electrons in an atom of...
- 14M.2.sl.TZ2.2b: The relative atomic mass of boron is 10.8, to three significant figures. Calculate the...
- 14M.2.sl.TZ2.2c: Isotopes of boron containing 7 and 8 neutrons also exist. Suggest why releasing isotopes...
- 14N.2.hl.TZ0.8a: (i) Calculate the relative atomic mass of this sample of magnesium correct to two decimal...
- 14N.1.sl.TZ0.5: Which ion will show the least deflection in a mass spectrometer? A. ...
- 14N.2.sl.TZ0.6a.i: Calculate the relative atomic mass of this sample of magnesium correct to two decimal places.
- 14N.2.sl.TZ0.6a.iii: Predict the relative atomic radii of the three magnesium isotopes, giving your reasons.
- 13N.1.hl.TZ0.4: What are the numbers of neutrons and electrons in the iodine ion,...
- 13N.2.hl.TZ0.8f.i: Outline why there are molecules with different molar masses.
- 13N.1.sl.TZ0.6: What are the numbers of neutrons and electrons in the iodine ion,...
- 13N.2.sl.TZ0.6f.i: Outline why there are molecules with different molar masses.
- 13M.1.sl.TZ1.6: Which statements about the isotopes of an element are correct? I. They have the same...
- 13M.2.sl.TZ1.6a.i: Define the term isotopes of an element.
- 13M.2.sl.TZ1.6a.ii: Calculate the percentage abundance of each isotope, given that the relative atomic mass of B...
- 13M.1.sl.TZ2.6: Which is an isotope of 24Mg? A. \(_{{\text{11}}}^{{\text{24}}}{\text{Na}}\) B. ...
- 13M.2.sl.TZ2.6a.i: Define the term isotopes of an element.
- 13M.2.sl.TZ2.6a.ii: Calculate the number of protons, neutrons and electrons in the isotopes 35Cl and 37Cl.
- 13M.2.sl.TZ2.6a.iii: Using the mass numbers of the two isotopes and the relative atomic mass of chlorine from...
- 12N.1.sl.TZ0.5: What is the correct number of each particle in an oxygen ion,...
- 12N.2.sl.TZ0.4a: (i) Define the terms atomic number, mass number and isotopes of an element. Atomic...
- 10N.1.sl.TZ0.6: Which statement about the species \(^{{\text{63}}}{\text{C}}{{\text{u}}^{2 + }}\) and...
- 10N.1.sl.TZ0.7: Which statement about the isotopes of an element is correct? A. They have the same mass...
- 10N.2.sl.TZ0.3b: A sample of iron has the following isotopic composition by mass. Calculate the relative...
- 10N.2.sl.TZ0.3c: Calculate the number of electrons in the ion \(^{{\text{56}}}{\text{F}}{{\text{e}}^{2 + }}\).
- 10M.2.sl.TZ1.4c: The relative atomic mass of naturally occurring copper is 63.55. Calculate the abundances of...
- 10M.1.sl.TZ2.6: How many electrons does the ion \(_{{\text{15}}}^{{\text{31}}}{{\text{P}}^{3 - }}\)...
- 09M.2.sl.TZ1.5a.iii: Strontium exists as four naturally-occurring isotopes. Calculate the relative atomic mass of...
- 09M.1.hl.TZ2.5: The table below shows the number of protons, neutrons and electrons present in five...
- 09M.1.sl.TZ2.5: What is the atomic number of a neutral atom which has 51 neutrons and 40 electrons? A. ...
- 09M.2.sl.TZ2.7a.i: Define the term isotopes.
- 09M.1.sl.TZ2.6: What is the relative atomic mass of an element with the following mass spectrum? A. ...
- 09M.2.sl.TZ2.7a.ii: A sample of silicon contains three isotopes. Calculate the relative atomic mass of silicon...
- 11M.2.hl.TZ1.3b: Deduce the numbers of protons and electrons in the ion \({\text{C}}{{\text{o}}^{2 + }}\).
- 11M.1.hl.TZ1.6: Which quantities are the same for all atoms of chlorine? I. Number of protons II. ...
- 11M.1.sl.TZ1.6: Which statement about the numbers of protons, electrons and neutrons in an atom is always...
- 11M.2.sl.TZ1.2b: Deduce the numbers of protons and electrons in the \({{\text{K}}^ + }\) ion.
- 11M.2.sl.TZ1.7f: Silicon has three stable isotopes, \(^{{\text{28}}}{\text{Si}}\),...
- 11M.1.hl.TZ2.4: Consider the relative abundance of the isotopes of element X. What is the relative atomic...
- 11M.2.hl.TZ2.2c.i: Calculate the percentage of each isotope in pure antimony. State your answers to three...
- 11M.2.hl.TZ2.2c.iii: State the number of neutrons present in an atom of \(^{{\text{121}}}{\text{Sb}}\).
- 11M.1.sl.TZ2.6: Which statements about the isotopes of chlorine, \(_{{\text{17}}}^{{\text{35}}}{\text{Cl}}\)...
- 11M.2.sl.TZ2.3a: Calculate the percentage of each isotope in pure rubidium. State your answers to...
- 11M.2.sl.TZ2.3c: State the number of electrons and the number of neutrons present in an atom of...
- 12M.1.sl.TZ2.6: Which subatomic particles are located in the nucleus of an atom? A. Protons and...
- 12M.2.sl.TZ2.4a.ii: Determine the number of neutrons in one atom of iodine-131.
- 11N.1.sl.TZ0.5: A sample of zinc has the following composition: What is the relative atomic mass of the...
- 11N.2.sl.TZ0.2a: Deduce the missing information and complete the following table.

