| Date | May 2015 | Marks available | 3 | Reference code | 15M.2.sl.TZ1.4 |
| Level | SL | Paper | 2 | Time zone | TZ1 |
| Command term | Calculate and State | Question number | 4 | Adapted from | N/A |
Question
State the relative mass and charge of the subatomic particles of an atom.
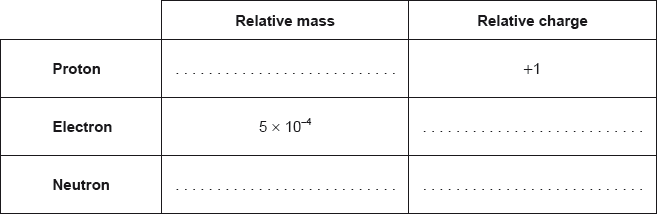
(i) Calculate the number of neutrons and electrons in one atom of \(^{{\text{65}}}{\text{Cu}}\).
Neutrons:
Electrons:
(ii) State one difference in the physical properties of the isotopes \(^{{\text{63}}}{\text{Cu}}\) and \(^{{\text{65}}}{\text{Cu}}\) and explain why their chemical properties are the same.
Physical:
Chemical:
Describe the bonding in solid copper.
Suggest two properties of copper that make it useful and economically important.
Markscheme
 ;
;
Award [2] for all four correct.
Award [1] for two or three correct.
(i) Neutrons: 36 and Electrons: 29;
(ii) Physical:
\(^{{\text{63}}}{\text{Cu}}\) lower boiling point/melting point/density/greater rate of diffusion than \(^{{\text{65}}}{\text{Cu}}\);
Accept converse arguments.
Do not accept “different mass”.
Chemical:
(properties identical because) same electron configuration/arrangement of electrons;
Accept “same number of protons and electrons”.
Do not accept “same number of electrons” OR “same valence (electrons)”
OR “same atomic number” only.
electrostatic attraction;
between (a lattice of) cations/positive ions and delocalized/sea of electrons;
Do not award any mark for only stating “metallic bonding”.
Award [1] for any two of:
malleable / ductile / conducts electricity / conducts heat / durable / strong / resistant to corrosion / low reactivity;
Examiners report
This question focused on atomic structure and metals was very accessible. Many students could correctly state relative mass and charge of the subatomic particles but it was disappointing to see the number of students who could not. Units were not penalized which meant that more students gained marks than would otherwise have been the case. Many students could calculate the protons and neutrons in the copper isotope but few could explain how the physical properties would vary (mass is not a property) and few could clearly explain why the chemical properties were identical. Explanations were often far too vague. Two properties of copper were asked for in and many could give one, again answers were often very vague, such as “it conducts” without specifying what it was conducting.
This question focused on atomic structure and metals was very accessible. Many students could correctly state relative mass and charge of the subatomic particles but it was disappointing to see the number of students who could not. Units were not penalized which meant that more students gained marks than would otherwise have been the case. Many students could calculate the protons and neutrons in the copper isotope but few could explain how the physical properties would vary (mass is not a property) and few could clearly explain why the chemical properties were identical. Explanations were often far too vague. Two properties of copper were asked for in and many could give one, again answers were often very vague, such as “it conducts” without specifying what it was conducting.
This question focused on atomic structure and metals was very accessible. Many students could correctly state relative mass and charge of the subatomic particles but it was disappointing to see the number of students who could not. Units were not penalized which meant that more students gained marks than would otherwise have been the case. Many students could calculate the protons and neutrons in the copper isotope but few could explain how the physical properties would vary (mass is not a property) and few could clearly explain why the chemical properties were identical. Explanations were often far too vague. Two properties of copper were asked for in and many could give one, again answers were often very vague, such as “it conducts” without specifying what it was conducting.
This question focused on atomic structure and metals was very accessible. Many students could correctly state relative mass and charge of the subatomic particles but it was disappointing to see the number of students who could not. Units were not penalized which meant that more students gained marks than would otherwise have been the case. Many students could calculate the protons and neutrons in the copper isotope but few could explain how the physical properties would vary (mass is not a property) and few could clearly explain why the chemical properties were identical. Explanations were often far too vague. Two properties of copper were asked for in and many could give one, again answers were often very vague, such as “it conducts” without specifying what it was conducting.

