| Date | May 2009 | Marks available | 2 | Reference code | 09M.2.hl.TZ1.9 |
| Level | HL | Paper | 2 | Time zone | TZ1 |
| Command term | Draw | Question number | 9 | Adapted from | N/A |
Question
But-1-ene and 1-aminobutane (1-butylamine) can both be prepared from 1-bromobutane.
2-bromobutane and 2-bromo-2-methylpropane are two isomers of 1-bromobutane.
Identify the type of reaction and explain the mechanism for the preparation of but-1-ene from 1-bromobutane using curly arrows to represent the movement of electron pairs.
State the equation (using structural formulas) for the preparation of 1-aminobutane from 1-bromobutane. State the necessary reagents and conditions of the reaction.
Explain the mechanism for the preparation of 1-aminobutane from 1-bromobutane using curly arrows to represent the movement of electron pairs.
Draw the structures of the two mirror images of the isomer that can exhibit optical isomerism.
Describe how the two optical isomers can be distinguished practically using plane-polarized light.
Explain why the mechanism of the reaction will be different if 1-bromobutane is replaced by 2-bromo-2-methylpropane to form 2-amino-2-methylpropane in the reaction in part (a) (iv).
Markscheme
elimination reaction ;
Then accept either E1 or E2 mechanism.
E1
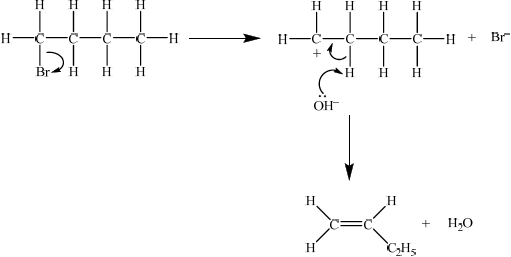
curly arrow showing bromine leaving the halogenoalkane;
\({\text{O}}{{\text{H}}^ - }\) acting as base on the intermediate carbocation;
E2
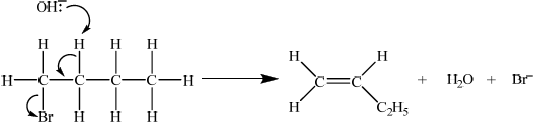
curly arrow showing\({\text{O}}{{\text{H}}^ - }\) acting as base on H bonded to C;
concerted curly arrows showing Br leaving C–Br;
\({\text{C}}{{\text{H}}_3}{\text{C}}{{\text{H}}_2}{\text{C}}{{\text{H}}_2}{\text{C}}{{\text{H}}_2}{\text{Br}} + {\text{N}}{{\text{H}}_3} \to {\text{C}}{{\text{H}}_3}{\text{C}}{{\text{H}}_2}{\text{C}}{{\text{H}}_2}{\text{C}}{{\text{H}}_2}{\text{N}}{{\text{H}}_2} + {\text{HBr}}\);
ammonia/\({\text{N}}{{\text{H}}_{\text{3}}}\);
warm / excess ammonia (to prevent secondary amines etc.);
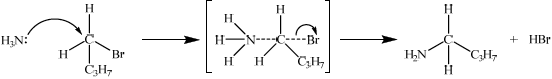
curly arrow from ammonia (to form transition state);
correct transition state;
curly arrow from bond to Br atom in either the first or second step;
formation of HBr and organic product;
Accept a second molecule of NH3 removing H+ from the transition state to give NH4+ and Br– as products.
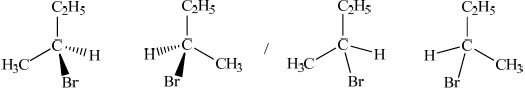
Award [1] for correct structure and [1] for correct 3-D representation of both enantiomers.
polarimeter (to measure angle of rotation);
the plane of plane-polarized light rotates in opposite directions (by the different enantiomers);
2-bromo-2-methylpropane is tertiary / 1-bromobutane is primary;
2-bromo-2-methylpropane goes by SN1 / 1-bromobutane by \({{\text{S}}_{\text{N}}}{\text{2}}\);
intermediate carbocation more stable for tertiary;
no space around tertiary carbon for five groups (in \({{\text{S}}_{\text{N}}}{\text{2}}\) transition state);
Examiners report
This question was the least popular of the Section B questions. Some candidates were very well prepared and scored well, while many struggled to write correct mechanisms with curly arrows in the right place. For a small number of candidates, all parts of the question other than the identification of a functional group proved very difficult. Additionally, in part (a), few candidates knew the details of the reagents and conditions for a range of reaction types.
This question was the least popular of the Section B questions. Some candidates were very well prepared and scored well, while many struggled to write correct mechanisms with curly arrows in the right place. For a small number of candidates, all parts of the question other than the identification of a functional group proved very difficult. Additionally, in part (a), few candidates knew the details of the reagents and conditions for a range of reaction types.
This question was the least popular of the Section B questions. Some candidates were very well prepared and scored well, while many struggled to write correct mechanisms with curly arrows in the right place. For a small number of candidates, all parts of the question other than the identification of a functional group proved very difficult. Additionally, in part (a), few candidates knew the details of the reagents and conditions for a range of reaction types.
In part (b), very few candidates could draw three-dimensional structures of optical isomers, although many gained a mark for correctly identifying the structure which could have enantiomers.
Few mentioned a polarimeter to distinguish between the two optical isomers, although several described in clear detail a practical method to do so.

