| Date | May 2010 | Marks available | 7 | Reference code | 10M.2.hl.TZ2.5 |
| Level | HL | Paper | 2 | Time zone | TZ2 |
| Command term | Describe, Draw, Identify, and Name | Question number | 5 | Adapted from | N/A |
Question
Existence of isomers leads to diversity of organic compounds.
(a) Describe what is meant by the term stereoisomers.
(b) 1,3-dichlorocyclobutane exists as geometrical isomers, a form of stereoisomers.
(i) Draw and name the two geometrical isomers of 1,3-dichlorocyclobutane.
(ii) Identify the isomer with the higher boiling point and explain your reasoning.
Markscheme
(a) compounds with same structural formula;
Do not allow “same molecular or chemical formula”.
but different arrangement of atoms in space/spatial arrangement;
(b) (i)
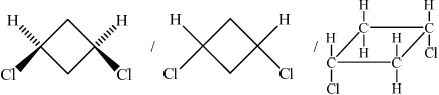 ;
;
Cis(-1,3-dichlorocyclobutane)
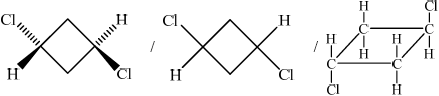 ;
;
Trans(-1,3-dichlorocyclobutane)
Need clear cis/trans structure and name for each mark.
(ii) cis (higher boiling point);
cis (more) polar / trans non-polar/less polar;
cis experiences stronger (permanent) dipole-dipole interaction / trans experiences no/(much) less dipole-dipole interaction;
Do not accept just strong forces without reference to dipole-dipole interaction.
Examiners report
Only a handful of candidates gave the correct definition of the term stereoisomers. Some stated that these had the same chemical or molecular formula with no reference to structural formula. Stereoisomers are compounds with the same structural formula, but with a different arrangement of the atoms in space. A majority of candidates drew correct formulas of the two geometrical isomers of 1,3-dichlorocyclobutane, but some missed the names of the compounds. Even when the notion of cis and trans seemed to be generally understood, the poor representation of molecules proved challenging for some where difference between the 2 isomers drawn was not at all clear.
A few candidates did not realise that the compound was cyclobutane and not straight chain butane. Nomenclature also emerged as a hindrance in the correct grasp of the topic with some candidates showing structures that had little resemblance to the names.
A good number of candidates identified the cis isomer as having the higher boiling point because it is more polar and experiences stronger dipole-dipole interactions between the molecules. Many candidates failed to provide enough details for the type of intermolecular interaction. A number of candidates incorrectly identified the trans as the polar molecule with the higher melting. Quite a few of the weaker candidates used arguments in terms of packing of the molecule and failed to score any mark.

