| Date | May 2014 | Marks available | 6 | Reference code | 14M.2.hl.TZ1.8 |
| Level | HL | Paper | 2 | Time zone | TZ1 |
| Command term | Draw, Explain, and Outline | Question number | 8 | Adapted from | N/A |
Question
\({\text{25.0 c}}{{\text{m}}^{\text{3}}}\) of \({\text{0.200 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\) ethanoic acid was added to \({\text{30.0 c}}{{\text{m}}^{\text{3}}}\) of a \({\text{0.150 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\) sodium hydrogencarbonate solution, \({\text{NaHC}}{{\text{O}}_{\text{3}}}{\text{(aq)}}\).
The molar mass of a volatile organic liquid, X, can be determined experimentally by allowing it to vaporize completely at a controlled temperature and pressure. 0.348 g of X was injected into a gas syringe maintained at a temperature of 90 °C and a pressure of \(1.01 \times {10^5}{\text{ Pa}}\). Once it had reached equilibrium, the gas volume was measured as \({\text{95.0 c}}{{\text{m}}^{\text{3}}}\).
Bromoethane, \({\text{C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{Br}}\), undergoes a substitution reaction to form ethylamine, \({\text{C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{N}}{{\text{H}}_{\text{2}}}\).
Many organic compounds exist as stereoisomers.
Outline how electrical conductivity can be used to distinguish between a \({\text{0.200 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\) solution of ethanoic acid, \({\text{C}}{{\text{H}}_{\text{3}}}{\text{COOH}}\), and a \({\text{0.200 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\) solution of hydrochloric acid, HCl.
(i) State an equation for the reaction of ethanoic acid with a solution of sodium hydrogencarbonate.
(ii) Determine which is the limiting reagent. Show your working.
(iii) Calculate the mass, in g, of carbon dioxide gas produced.
(i) Determine the amount, in mol, of X in the gas syringe.
(ii) Calculate the molar mass of X.
Deduce the mechanism for the reaction using equations and curly arrows to represent the movement of electron pairs.
(i) Outline the meaning of the term stereoisomers.
(ii) Draw the structures of the two stereoisomers of dichloroethene, \({{\text{C}}_{\text{2}}}{{\text{H}}_{\text{2}}}{\text{C}}{{\text{l}}_{\text{2}}}\).
(iii) Explain why this type of stereoisomerism exists in \({{\text{C}}_{\text{2}}}{{\text{H}}_{\text{2}}}{\text{C}}{{\text{l}}_{\text{2}}}\).
(iv) Draw the structures of the two stereoisomers of 1-chloro-1-fluoroethane, \({{\text{C}}_{\text{2}}}{{\text{H}}_{\text{4}}}{\text{FCl}}\), showing the relationship between them.
(v) Outline how the two isomers of \({{\text{C}}_{\text{2}}}{{\text{H}}_{\text{4}}}{\text{FCl}}\) could be distinguished from each other.
Markscheme
HCl is a strong acid and \({\text{C}}{{\text{H}}_3}{\text{COOH}}\) is a weak acid so HCl has higher conductivity / HCl dissociates completely in water and \({\text{C}}{{\text{H}}_3}{\text{COOH}}\) does not, so HCl has higher conductivity / HCl is a stronger acid (than \({\text{C}}{{\text{H}}_{\text{3}}}{\text{COOH}}\)) so has higher \({\text{[}}{{\text{H}}^ + }{\text{]}}\) and higher conductivity;
(i) \({\text{C}}{{\text{H}}_3}{\text{COOH(aq)}} + {\text{HCO}}_3^ - {\text{(aq)}} \to {\text{C}}{{\text{H}}_3}{\text{CO}}{{\text{O}}^ - }{\text{(aq)}} + {{\text{H}}_2}{\text{O(l)}} + {\text{C}}{{\text{O}}_2}{\text{(g)}}\);
Accept NaHCO3(aq) and CH3COONa (aq) instead of ions.
Ignore state symbols.
(ii) \(n({\text{C}}{{\text{H}}_3}{\text{COOH}}) = 0.00500{\text{ (mol)}}\) and \(n{\text{(NaHC}}{{\text{O}}_3}{\text{)}} = 0.00450{\text{ (mol)}}\);
\({\text{NaHC}}{{\text{O}}_3}\) is limiting;
(iii) \(n{\text{(C}}{{\text{O}}_2}{\text{)}} = n{\text{(NaHC}}{{\text{O}}_{\text{3}}}{\text{)}} = 0.00450{\text{ (mol)}}\);
\(m{\text{(C}}{{\text{O}}_2}{\text{)}} = 0.00450 \times 44.01 = 0.198{\text{(g)}}\);
Award [2] for correct final answer.
(i) \(T = 363{\text{ K}}\) and \(V = 9.50 \times {10^{ - 5}}{\text{ }}{{\text{m}}^3}\);
Accept V = 9.5 \( \times \) 10–2 dm3 if P is used as 101 kPa in calculation.
\(n = \frac{{PV}}{{RT}} = \frac{{1.01 \times {{10}^5} \times 9.50 \times {{10}^{ - 5}}}}{{8.31 \times 363}}\);
\( = 3.18 \times {10^{ - 3}}{\text{ (mol)}}\);
Award [3] for correct final answer.
(ii) \(M - \left( {\frac{m}{n} = \frac{{0.348}}{{3.18 \times {{10}^{ - 3}}}} = } \right){\text{ }}109{\text{ (g}}\,{\text{mo}}{{\text{l}}^{ - {\text{1}}}}{\text{)}}\);
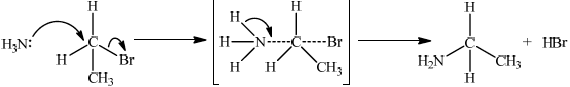
curly arrow going from lone pair on N in \({\text{N}}{{\text{H}}_{\text{3}}}\) to C;
curly arrow showing Br leaving;
Accept curly arrow going from bond between C and Br to Br on 1-bromoethane or on the transition state.
representation of transition state showing square brackets, two partial bonds and curly arrow going from NH bond to NC partial bond/curly arrow going from NH bond to N;
Do not penalize if NH3 and Br are not at 180° to each other.
Do not award M3 if NH3—C bond is represented.
(i) compounds with same structural formula but different arrangements of atoms in space;
(ii)
The two structures must be clear 3D representations of mirror images.
Tapered (wedge/dash) notation not necessary.
(iii) restricted rotation around (C=C) double bond;
(iv)
(v) the two enantiomers rotate the plane of plane-polarized light by equal amounts, but in opposite directions;
using a polarimeter;
Examiners report
Poorly constructed symbolic equations on what should be relatively simple reactions once again impeded candidates from credit. The use of \(pV = nRT\) often scored for error carried forward even when they lost the first mark from incorrect use of units for pressure. The attempts at the \({{\text{S}}_{\text{N}}}{\text{2}}\) mechanisms were generally poor, with errors both in the attacking nucleophile, and the sloppy use of curly arrows which indicate that many students have a basic lack of understanding about what they represent. While candidates could score the first two marks, the third mark was almost never awarded. Conditions and reagents in d(ii) and d(iii) were rarely known, and definitions of stereoisomers and the representation of 3D structures was disappointing.
Poorly constructed symbolic equations on what should be relatively simple reactions once again impeded candidates from credit. The use of \(pV = nRT\) often scored for error carried forward even when they lost the first mark from incorrect use of units for pressure. The attempts at the \({{\text{S}}_{\text{N}}}{\text{2}}\) mechanisms were generally poor, with errors both in the attacking nucleophile, and the sloppy use of curly arrows which indicate that many students have a basic lack of understanding about what they represent. While candidates could score the first two marks, the third mark was almost never awarded. Conditions and reagents in d(ii) and d(iii) were rarely known, and definitions of stereoisomers and the representation of 3D structures was disappointing.
Poorly constructed symbolic equations on what should be relatively simple reactions once again impeded candidates from credit. The use of \(pV = nRT\) often scored for error carried forward even when they lost the first mark from incorrect use of units for pressure. The attempts at the \({{\text{S}}_{\text{N}}}{\text{2}}\) mechanisms were generally poor, with errors both in the attacking nucleophile, and the sloppy use of curly arrows which indicate that many students have a basic lack of understanding about what they represent. While candidates could score the first two marks, the third mark was almost never awarded. Conditions and reagents in d(ii) and d(iii) were rarely known, and definitions of stereoisomers and the representation of 3D structures was disappointing.
Poorly constructed symbolic equations on what should be relatively simple reactions once again impeded candidates from credit. The use of \(pV = nRT\) often scored for error carried forward even when they lost the first mark from incorrect use of units for pressure. The attempts at the \({{\text{S}}_{\text{N}}}{\text{2}}\) mechanisms were generally poor, with errors both in the attacking nucleophile, and the sloppy use of curly arrows which indicate that many students have a basic lack of understanding about what they represent. While candidates could score the first two marks, the third mark was almost never awarded. Conditions and reagents in d(ii) and d(iii) were rarely known, and definitions of stereoisomers and the representation of 3D structures was disappointing.
Poorly constructed symbolic equations on what should be relatively simple reactions once again impeded candidates from credit. The use of \(pV = nRT\) often scored for error carried forward even when they lost the first mark from incorrect use of units for pressure. The attempts at the \({{\text{S}}_{\text{N}}}{\text{2}}\) mechanisms were generally poor, with errors both in the attacking nucleophile, and the sloppy use of curly arrows which indicate that many students have a basic lack of understanding about what they represent. While candidates could score the first two marks, the third mark was almost never awarded. Conditions and reagents in d(ii) and d(iii) were rarely known, and definitions of stereoisomers and the representation of 3D structures was disappointing.

