| Date | November 2009 | Marks available | 1 | Reference code | 09N.3.hl.TZ0.F3 |
| Level | HL | Paper | 3 | Time zone | TZ0 |
| Command term | Explain | Question number | F3 | Adapted from | N/A |
Question
Different conventions are used to classify enantiomers. Orange and lemon peel each contain different enantiomers of the compound limonene. One of the enantiomers is represented below.

Identify the chiral centre in this enantiomer with an asterisk, *.
The \(( + )d\)-enantiomer has the characteristic smell of oranges and the \(( - )l\)-enantiomer has the characteristic smell of lemons. Explain the meaning of the \(( + )d\) and \(( - )l\) symbols used in this notation.
The R, S notation is also used. The \(( + )d\)-enantiomer is often described as R-limonene and the \(( - )l\)-enantiomer as S-limonene. Explain what is meant by the R, S notation and state whether the structure shown is R or S.
Markscheme
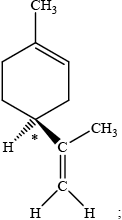
dextro/\(d\) and levo/\(l\) refer to right and left-handed / clockwise and anti-clockwise rotation of plane polarized light;
clockwise and anti-clockwise sequence of prioritized atoms (working from high to low atomic numbers) / OWTTE;
Allow absolute configuration of enantiomers.
Allow convention for labelling chiral carbon atoms using the Cahn-Ingold-Prelog notation.
S;
Examiners report
In (a), surprisingly a number of students were not able to identify the chiral centre.
Although some knew what \(d\) and \(l\) referred to, a number did not refer to plane-polarized light.
R and S notation was not understood and only the best candidates were able to determine the structure as S. Some simple class exercises on chiral centres, \(d\) and \(d\) notation and R and S notation using a number of simple molecules would greatly improve student understanding of this topic.

