| Date | November 2010 | Marks available | 2 | Reference code | 10N.2.hl.TZ0.5 |
| Level | HL | Paper | 2 | Time zone | TZ0 |
| Command term | Draw | Question number | 5 | Adapted from | N/A |
Question
Consider the following sequence of reactions.
\[{\text{RC}}{{\text{H}}_3}\xrightarrow{{reaction 1}}{\text{RC}}{{\text{H}}_2}{\text{Br}}\xrightarrow{{reaction 2}}{\text{RC}}{{\text{H}}_2}{\text{OH}}\]
\({\text{RC}}{{\text{H}}_{\text{3}}}\) is an unknown alkane in which R represents an alkyl group.
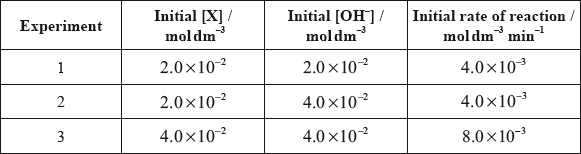
All the isomers can by hydrolysed with aqueous sodium hydroxide solution. When the reaction of one of these isomers, X, was investigated the following kinetic data were obtained.
The alkane contains 82.6% by mass of carbon. Determine its empirical formula, showing your working.
A 1.00 g gaseous sample of the alkane has a volume of 385 cm3 at standard temperature and pressure. Deduce its molecular formula.
State the reagent and conditions needed for reaction 1.
Reaction 1 involves a free-radical mechanism. Describe the stepwise mechanism, by giving equations to represent the initiation, propagation and termination steps.
The mechanism in reaction 2 is described as SN2. Explain the mechanism of this reaction using curly arrows to show the movement of electron pairs, and draw the structure of the transition state.
There are four structural isomers with the molecular formula \({{\text{C}}_{\text{4}}}{{\text{H}}_{\text{9}}}{\text{Br}}\). One of these structural isomers exists as two optical isomers. Draw diagrams to represent the three-dimensional structures of the two optical isomers.
(i) Deduce the rate expression for the reaction.
(ii) Determine the value of the rate constant for the reaction and state its units.
(iii) State the name of isomer X and explain your choice.
(iv) State equations for the steps that take place in the mechanism of this reaction and state which of the steps is slow and which is fast.
Markscheme
\({n_{\text{C}}} = \frac{{82.6}}{{12.01}} = 6.88\) and \({n_{\text{H}}} = \frac{{17.4}}{{1.01}} = 17.2\);
ratio is 1:2.5;
\({{\text{C}}_2}{{\text{H}}_5}\);
No penalty for using 12 and 1.
\(\left( {M = \frac{{22400}}{{385}}} \right) = 58.2/\left( {M = \frac{{mRT}}{{PV}}} \right) = 58.3\);
\({{\text{C}}_4}{{\text{H}}_{10}}\);
Br2/bromine ;
UV/ultraviolet light;
Accept hf/hv/sunlight.
initiation:
\({\text{B}}{{\text{r}}_2} \to {\text{2Br}} \bullet \);
propagation:
\({\text{Br}} \bullet + {\text{RC}}{{\text{H}}_3} \to {\text{HBr}} + {\text{RC}}{{\text{H}}_2} \bullet \);
\({\text{RC}}{{\text{H}}_2} \bullet + {\text{B}}{{\text{r}}_2} \to {\text{RC}}{{\text{H}}_2}{\text{Br}} + {\text{Br}} \bullet \);
termination: [1 max]
\({\text{Br}} \bullet + {\text{Br}} \bullet \to {\text{B}}{{\text{r}}_2}\);
\({\text{RC}}{{\text{H}}_2} \bullet + {\text{Br}} \bullet \to {\text{RC}}{{\text{H}}_2}{\text{Br}}\);
\({\text{RC}}{{\text{H}}_2} \bullet + {\text{RC}}{{\text{H}}_2} \bullet \to {\text{RC}}{{\text{H}}_2}{\text{C}}{{\text{H}}_2}{\text{R}}\);
Award [1] for any termination step.
Accept radical with or without throughout.
Do not penalise the use of an incorrect alkane in the mechanism.
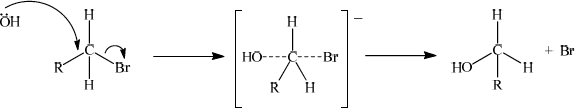
curly arrow going from lone pair/negative charge on O in OH– to C;
Do not allow curly arrow originating on H in OH–.
curly arrow showing Br leaving;
Accept curly arrow either going from bond between C and Br to Br in bromoethane or in the transition state.
representation of transition state showing negative charge, square brackets and partial bonds;
Do not penalize if HO and Br are not at 180° to each other.
Do not award M3 if OH ---- C bond is represented unless already penalised in M1.
Do not penalise the use of an incorrect alkyl chain in the mechanism.
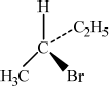 ;
;
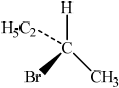 ;
;
First and second structures should be mirror images. Tetrahedral arrangement around carbon must be shown.
(i) order with respect to \({\text{O}}{{\text{H}}^ - } = {\text{0}}\);
order with respect to \({\text{X}} = 1\);
rate \( = k{\text{[X]}}\);
Award [3] for final correct answer.
(ii) 0.2(0);
\({\text{mi}}{{\text{n}}^{ - 1}}\);
(iii) 2-bromo-2-methyl-propane;
Do not penalize missing hyphens or added spaces.
Accept 2-methyl-2-bromopropane.
tertiary structure;
(iv) \({{\text{C}}_4}{{\text{H}}_9}{\text{Br}} \to {{\text{C}}_4}{\text{H}}_9^ + + {\text{B}}{{\text{r}}^ - }\) / in equation with curly arrows and slow;
\({{\text{C}}_4}{\text{H}}_9^ + + {\text{O}}{{\text{H}}^ - } \to {{\text{C}}_4}{{\text{H}}_9}{\text{OH}}\) / in equation with curly arrows and fast;
No penalty if primary structure is shown.
No credit for SN2 mechanism, except by ECF.
Examiners report
Although this was the least popular question in Section B there was generally a good level of performance. In (a) most candidates scored at least 2 out of 3 marks for calculating the empirical formula.
Many managed to give a correct molecular formula based on their background knowledge once they had determined the molar mass from the density calculation.
The conditions of free radical substitution were well known.
The mechanism of free radical substitution was well known.
The conditions and mechanism of free radical substitution were well known but the SN2 mechanism in (e) caused more problems.
Again the use of curly arrows proved to be difficult. In some case they originated from the H not the lone pair on O of the nucelophile, or were missing from the C – Br bond. Another common mistake was the omission of a negative charge from the transition state. As the attack of the nucleophile is on the opposite side of the carbon atom to the halogen leaving, the partial bonds in the transition state should be drawn at 180 degrees. Candidates were not penalised however if they failed to do this.
Most candidates were able to draw accurate 3D diagrams for the stereoisomers of 2-bromobutane, to deduce the rate expression from the experimental data presented in (g), and correctly identify X as having a tertiary structure. It was also pleasing to see that most were able to describe the SN1 mechanism.

