| Date | May 2010 | Marks available | 2 | Reference code | 10M.2.hl.TZ2.2 |
| Level | HL | Paper | 2 | Time zone | TZ2 |
| Command term | Draw and Explain | Question number | 2 | Adapted from | N/A |
Question
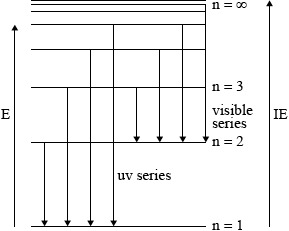
On the above diagram, draw the line that corresponds to the first ionization energy of hydrogen and explain your reasoning.
Markscheme
for showing the energy to remove electron from \({\text{n}} = 1\) to \({\text{n}} = \infty \) on the above diagram;
to ionize an element, electron must be removed from the atom/no longer under influence of nucleus/removed beyond \({\text{n}} = \infty \) / OWTTE;
Examiners report
Candidates in some schools, however, appeared not to have encountered these ideas at all. Common errors were to label the first energy level as\({\text{n}} = 0\) rather than\({\text{n}} = 1\) and to only include one transition for each series. Sometimes the arrows showing the transitions were shown from the bottom up.
While more candidates managed to obtain at least a mark with regard to the first ionization energy of hydrogen, it was less common to find the correct graphical representation of the first IE with diagrams which often were unrelated.

