| Date | November 2010 | Marks available | 8 | Reference code | 10N.2.hl.TZ0.4 |
| Level | HL | Paper | 2 | Time zone | TZ0 |
| Command term | Define and Explain | Question number | 4 | Adapted from | N/A |
Question
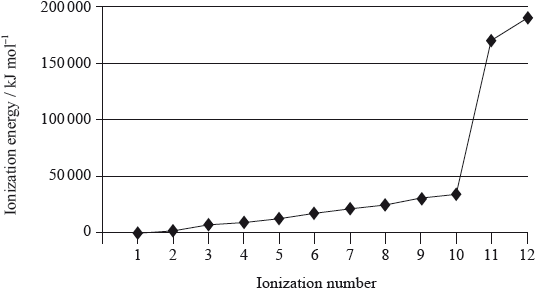
Magnesium is the eighth most abundant element in the earth’s crust. The successive ionization energies of the element are shown below.
Magnesium can be produced from the electrolysis of molten magnesium chloride, MgCl2.
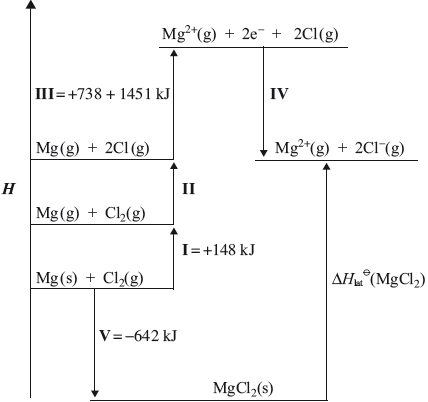
The lattice enthalpy of magnesium chloride can be calculated from the Born-Haber cycle shown below.
(i) Define the term first ionization energy and state the equation for the first ionization of magnesium.
(ii) Explain the general increase in successive ionization energies of the element.
(iii) Explain the large increase between the tenth and eleventh ionization energies.
(i) Explain how molten magnesium chloride conducts an electric current.
(ii) Identify the electrode where oxidation occurs during electrolysis of molten magnesium chloride and state an equation for the half-reaction.
(iii) Explain why magnesium is not formed during the electrolysis of aqueous magnesium chloride solution.
(i) Identify the enthalpy changes labelled by I and V in the cycle.
(ii) Use the ionization energies given in the cycle above and further data from the Data Booklet to calculate a value for the lattice enthalpy of magnesium chloride.
(iii) The theoretically calculated value for the lattice enthalpy of magnesium chloride is +2326 kJ. Explain the difference between the theoretically calculated value and the experimental value.
(iv) The experimental lattice enthalpy of magnesium oxide is given in Table 13 of the Data Booklet. Explain why magnesium oxide has a higher lattice enthalpy than magnesium chloride.
(i) State whether aqueous solutions of magnesium oxide and magnesium chloride are acidic, alkaline or neutral.
(ii) State an equation for the reaction between magnesium oxide and water.
Markscheme
(i) energy (per mole) needed to remove one/first/most loosely bound electron from a (neutral) atom;
in the gaseous state;
\({\text{Mg(g)}} \to {\text{M}}{{\text{g}}^ + }{\text{(g)}} + {{\text{e}}^ - }\);
Gaseous state symbols needed.
Accept e instead of e–.
Only penalize omission of gas phase once in either the second marking point or the third marking point.
(ii) successive electrons (are more difficult to remove because each is) taken from more positively charged ion/ OWTTE;
increased electrostatic attraction;
(iii) 10th electron comes from 2nd energy level/\(n = 2\) and 11th electron comes from 1st first energy level/\(n = 1\) / OWTTE;
electron in 1st energy level closer to nucleus;
electron in 1st energy level not shielded by inner electrons / exposed to greater effective nuclear charge;
(i) contains ions which are free to move (only) in molten state;
\({\text{M}}{{\text{g}}^{2 + }}\) move to cathode/negative electrode and \({\text{C}}{{\text{l}}^ - }\) move to anode/positive electrode / OWTTE;
(ii) anode/positive electrode;
\({\text{2C}}{{\text{l}}^ - } \to {\text{C}}{{\text{l}}_2} + {\text{2}}{{\text{e}}^ - }/{\text{C}}{{\text{l}}^ - } \to \frac{1}{2}{\text{C}}{{\text{l}}_2}+{{\text{e}}^ - }\);
Accept e instead of e–.
Do not accept \(C{l^ - } \to Cl + {e^ - }\).
Ignore state symbols.
(iii) magnesium has large negative electrode potential / \({E^\Theta }\);
reduction of \({{\text{H}}_2}{\text{O/}}{{\text{H}}^ + }\) to \({{\text{H}}_2}\) has less negative electrode potential;
\({\text{M}}{{\text{g}}^{2 + }}\) not readily reduced (in comparison to \({{\text{H}}_2}{\text{O}}\));
if formed, magnesium would (immediately) react with water to form \({\text{M}}{{\text{g}}^{2 + }}\);
magnesium more reactive than hydrogen;
Do not accept Mg too reactive.
(i) I:
atomization/sublimation (of Mg) / \(\Delta H_{{\text{atomization}}}^\Theta {\text{(Mg)}}\) / \(\Delta H_{{\text{sublimation}}}^\Theta {\text{(Mg)}}\);
V:
enthalpy change of formation of \({\text{(MgC}}{{\text{l}}_2}{\text{)}}\) / \(\Delta H_{{\text{formation}}}^\Theta {\text{(MgC}}{{\text{l}}_2}{\text{)}}\);
(ii) Energy value for II:
\( + 243\);
Energy value for III:
\(738 + 1451 = 2189\);
Energy value for IV:
\(2( - 349)\);
\(\Delta H_{{\text{lat}}}^\Theta {\text{(MgC}}{{\text{l}}_2}{\text{)}} = 642 + 148 + 243 + 2189 - 2(349) = ( - )2524{\text{ (kJ)}}\);
(iii) theoretical value assumes ionic model;
experimental value greater due to (additional) covalent character;
(iv) oxide greater charge;
oxide smaller radius;
Accept opposite arguments.
(i) \({\text{MgC}}{{\text{l}}_2}\) (weakly) acidic and MgO alkaline;
(ii) \({\text{MgO}} + {{\text{H}}_2}{\text{O}} \to {\text{Mg(OH}}{{\text{)}}_2}\);
Ignore state symbols.
Examiners report
In (a) several candidates failed to mention atoms in their definition of first ionization energy and others neglected to state that the gaseous state is involved. Only the strongest students mentioned the electrostatic nature of the attraction between the nucleus and the electrons in explaining trends in ionisation energies. Several candidates lost a mark in explaining the increase between the tenth and eleventh ionisation energies as their arguments were incomplete, with no reference to the change from\(n = 2\) energy level to the\(n = 1\) level.
In (b) many candidates stated that free electrons rather than ions were responsible for the conductivity of magnesium chloride and others did not refer to the movement of both \({\text{M}}{{\text{g}}^{2 + }}\) and \({\text{C}}{{\text{l}}^ - }\) ions. The anode was generally identified as the electrode where oxidation occurs but some had difficulties giving the balanced equation for the half reaction. Only the strongest candidates were able to explain why magnesium is not formed during the electrolysis of aqueous solutions.
In (c) most candidates were familiar with the enthalpy changes of atomization and formation but some struggled with the Born Haber Cycle. Only the strongest candidates were able to relate differences in experimental and theoretical lattice energies to the covalent character of the solid with a significant number mistakenly giving “heat loss” as the reason for the difference.
In (d) many were able to correctly describe the basic nature of magnesium oxide but the acidity of magnesium chloride was less well known. Some gave hydrogen gas as a product in the reaction between magnesium oxide and water.

