| Date | November 2014 | Marks available | 1 | Reference code | 14N.1.hl.TZ0.5 |
| Level | HL | Paper | 1 | Time zone | TZ0 |
| Command term | Question number | 5 | Adapted from | N/A |
Question
Successive ionization energies for an element, Z, are shown in the table below.
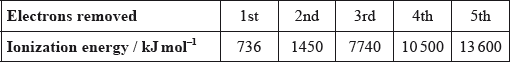
What is the most likely formula for the ion of Z?
A. \({{\text{Z}}^ + }\)
B. \({{\text{Z}}^{2 + }}\)
C. \({{\text{Z}}^{3 + }}\)
D. \({{\text{Z}}^{4 + }}\)
Markscheme
B
Examiners report
[N/A]

