| Date | May 2014 | Marks available | 2 | Reference code | 14M.2.hl.TZ1.5 |
| Level | HL | Paper | 2 | Time zone | TZ1 |
| Command term | Explain | Question number | 5 | Adapted from | N/A |
Question
The oxides and chlorides of period 3 elements exhibit periodicity.
Chlorine gas, \({\text{C}}{{\text{l}}_{\text{2}}}{\text{(g)}}\), is bubbled through separate solutions of aqueous bromine, \({\text{B}}{{\text{r}}_{\text{2}}}{\text{(aq)}}\), and potassium bromide, \({\text{KBr(aq)}}\).
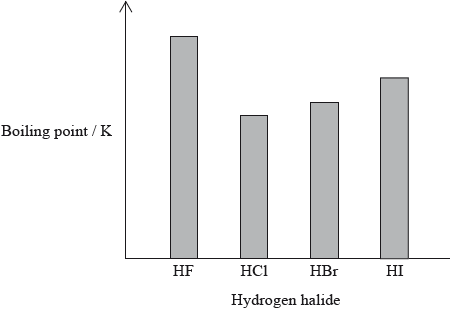
The hydrogen halides do not show perfect periodicity. A bar chart of boiling points shows that the boiling point of hydrogen fluoride, HF, is much higher than periodic trends would indicate.
Transition metals form complex ions which are usually coloured.
(i) State the changes in the acid-base nature of the oxides across period 3 (from \({\text{N}}{{\text{a}}_2}{\text{O}}\) to \({\text{C}}{{\text{l}}_{\text{2}}}{{\text{O}}_{\text{7}}}\)), including equations for the reactions of \({\text{N}}{{\text{a}}_2}{\text{O}}\) and \({\text{S}}{{\text{O}}_{\text{3}}}\) with water.
(ii) State whether or not molten aluminium chloride, \({\text{A}}{{\text{l}}_{\text{2}}}{\text{C}}{{\text{l}}_{\text{6}}}\), and molten aluminium oxide, \({\text{A}}{{\text{l}}_{\text{2}}}{{\text{O}}_{\text{3}}}\), conduct electricity. Explain this behaviour in terms of the structure and bonding of the two compounds.
(iii) State the equation for the reaction of \({\text{C}}{{\text{l}}_{\text{2}}}\) with water.
(i) Predict any changes that may be observed in each case.
\({\text{B}}{{\text{r}}_{\text{2}}}{\text{(aq)}}\):
\({\text{KBr(aq)}}\):
(ii) State the half-equations for the reactions that occur.
(i) Explain why the boiling point of HF is much higher than the boiling points of the other hydrogen halides.
(ii) Explain the trend in the boiling points of HCl, HBr and HI.
State the full electron configurations of Cr and \({\text{C}}{{\text{r}}^{3 + }}\).
Cr:
\({\text{C}}{{\text{r}}^{3 + }}\):
\({\text{C}}{{\text{r}}^{3 + }}\) ions and water molecules bond together to form the complex ion \({{\text{[Cr(}}{{\text{H}}_{\text{2}}}{\text{O}}{{\text{)}}_{\text{6}}}{\text{]}}^{3 + }}\).
Describe how the water acts and how it forms the bond, identifying the acid-base character of the reaction.
Explain why the \({{\text{[Cr(}}{{\text{H}}_{\text{2}}}{\text{O}}{{\text{)}}_{\text{6}}}{\text{]}}^{3 + }}\) ion is coloured.
Outline, including a relevant equation, whether the \({{\text{[Cr(}}{{\text{H}}_{\text{2}}}{\text{O}}{{\text{)}}_{\text{6}}}{\text{]}}^{3 + }}\) ion is acidic, basic or neutral.
Explain how the number of electrons in the outer main energy level of phosphorus, P, can be determined using the data of successive ionization energies.
Markscheme
(i) basic to acidic;
\({\text{N}}{{\text{a}}_{\text{2}}}{\text{O(s)}} + {{\text{H}}_{\text{2}}}{\text{O(l)}} \to {\text{2NaOH(aq)}}\);
\({\text{S}}{{\text{O}}_3}{\text{(g)}} + {{\text{H}}_2}{\text{O(l)}} \to {{\text{H}}_2}{\text{S}}{{\text{O}}_4}{\text{(aq)}}\);
Ignore state symbols.
(ii) molten \({\text{A}}{{\text{l}}_2}{\text{C}}{{\text{l}}_6}\) does not conduct electricity and molten \({\text{A}}{{\text{l}}_{\text{2}}}{{\text{O}}_{\text{3}}}\) does;
\({\text{A}}{{\text{l}}_2}{\text{C}}{{\text{l}}_6}\) is a covalent molecule and has no free charged particles to conduct electricity;
\({\text{A}}{{\text{l}}_2}{{\text{O}}_3}\) is ionic/has ions which are free to move when molten;
(iii) \({\text{C}}{{\text{l}}_2}{\text{(g)}} + {{\text{H}}_2}{\text{O(l)}} \rightleftharpoons {\text{HCl(aq)}} + {\text{HClO(aq)}}\);
Ignore state symbols.
Allow \( \to \).
(i) \({\text{B}}{{\text{r}}_2}{\text{(aq)}}\): no change;
\({\text{KBr(aq)}}\): colour change / from colourless to red/yellow/orange/brown;
(ii) \({\text{2B}}{{\text{r}}^ - }{\text{(aq)}} \to {\text{B}}{{\text{r}}_2}{\text{(aq)}} + {\text{2}}{{\text{e}}^ - }\);
\({\text{C}}{{\text{l}}_2}{\text{(g)}} + {\text{2}}{{\text{e}}^ - } \to {\text{2C}}{{\text{l}}^ - }{\text{(aq)}}\);
Ignore state symbols.
Accept e instead of e–.
(i) HF has hydrogen bonds (between molecules);
(ii) strength of van der Waals’/London/dispersion forces increases;
as mass/size/number of electrons of halogen atom/molecule increases;
Cr: \({\text{1}}{{\text{s}}^{\text{2}}}{\text{2}}{{\text{s}}^{\text{2}}}{\text{2}}{{\text{p}}^{\text{6}}}{\text{3}}{{\text{s}}^{\text{2}}}{\text{3}}{{\text{p}}^{\text{6}}}{\text{4}}{{\text{s}}^{\text{1}}}{\text{3}}{{\text{d}}^{\text{5}}}/{\text{1}}{{\text{s}}^{\text{2}}}{\text{2}}{{\text{s}}^{\text{2}}}{\text{2}}{{\text{p}}^{\text{6}}}{\text{3}}{{\text{s}}^{\text{2}}}{\text{3}}{{\text{p}}^{\text{6}}}{\text{3}}{{\text{d}}^{\text{5}}}{\text{4}}{{\text{s}}^{\text{1}}}\);
Cr3+: \({\text{1}}{{\text{s}}^{\text{2}}}{\text{2}}{{\text{s}}^{\text{2}}}{\text{2}}{{\text{p}}^{\text{6}}}{\text{3}}{{\text{s}}^{\text{2}}}{\text{3}}{{\text{p}}^{\text{6}}}{\text{3}}{{\text{d}}^{\text{3}}}\);
\({{\text{H}}_2}{\text{O}}\) is a ligand / has lone (electron) pair;
forms dative (covalent)/coordinate bond / donates a lone (electron) pair ;
ligand is Lewis base / \({\text{C}}{{\text{r}}^{3 + }}\) is Lewis acid;
\({\text{C}}{{\text{r}}^{3 + }}\) has partially filled d orbitals;
d orbitals split into two levels / three lower energy and two higher energy levels;
energy difference is in visible part of spectrum;
electrons absorb visible light / one colour/frequency/wavelength;
electron transitions occur from lower to higher energy level within d sub-level;
complementary colour/colour not absorbed is seen;
acidic because \({{\text{[Cr(}}{{\text{H}}_2}{\text{O}}{{\text{)}}_6}{\text{]}}^{3 + }}{\text{(aq)}} \to {{\text{[Cr(}}{{\text{H}}_2}{\text{O}}{{\text{)}}_5}{\text{(OH)]}}^{2 + }}{\text{(aq)}} + {{\text{H}}^ + }{\text{(aq)}}\);
Allow answers with further equations.
Accept any other valid equations.
Ignore state symbols.
successive ionization energy values increase with removal of each electron;
large increase in ionization energy when sixth electron is removed;
as electron is one energy level/shell closer to the nucleus;
Accept a suitably annotated diagram.
Examiners report
There appeared to be some significant gaps in knowledge within this question, the various parts either scored very well or not at all.
In a(ii) there was a poor understanding of the nature of bonding in aluminium chloride and aluminium oxide. Candidates are still confusing electrical conductivity in compounds with that in metals, and often refer to the inability to conduct being down to a lack of mobile electrons in compounds.
Balancing equations, both full, as in parts a(i) and a(iii), and half equations as in b(ii), showed poor knowledge both of the reactants and products and in the ability to balance them in both atoms and charge. It should be expected that higher level candidates would be comfortable with these processes. The ability to deduce and predict what they would see during a reaction is a skill required of all chemists, it was missing in the attempts to answer b(ii). Parts c and d(i), (ii) and (iii) showed good knowledge, but in part d(iv) the understanding of the acid nature of some d block complex ions was lacking. Part e was rarely given credit, as many appeared to misread the question, and discussed the changes in first ionisation energies across Period 3.
There appeared to be some significant gaps in knowledge within this question, the various parts either scored very well or not at all.
In a(ii) there was a poor understanding of the nature of bonding in aluminium chloride and aluminium oxide. Candidates are still confusing electrical conductivity in compounds with that in metals, and often refer to the inability to conduct being down to a lack of mobile electrons in compounds.
Balancing equation,s both full, as in parts a(i) and a(iii), and half equations as in b(ii), showed poor knowledge both of the reactants and products and in the ability to balance them in both atoms and charge. It should be expected that higher level candidates would be comfortable with these processes. The ability to deduce and predict what they would see during a reaction is a skill required of all chemists, it was missing in the attempts to answer b(ii). Parts c and d(i), ii) and iii) showed good knowledge, but in part d(iv) the understanding of the acid nature of some d block complex ions was lacking. Part e was rarely given credit, as many appeared to misread the question, and discussed the changes in first ionisation energies across Period 3.
There appeared to be some significant gaps in knowledge within this question, the various parts either scored very well or not at all.
In a(ii) there was a poor understanding of the nature of bonding in aluminium chloride and aluminium oxide. Candidates are still confusing electrical conductivity in compounds with that in metals, and often refer to the inability to conduct being down to a lack of mobile electrons in compounds.
Balancing equations, both full, as in parts a(i) and a(iii), and half equations as in b(ii), showed poor knowledge both of the reactants and products and in the ability to balance them in both atoms and charge. It should be expected that higher level candidates would be comfortable with these processes. The ability to deduce and predict what they would see during a reaction is a skill required of all chemists, it was missing in the attempts to answer b(ii). Parts c and d(i), ii) and iii) showed good knowledge, but in part d(iv) the understanding of the acid nature of some d block complex ions was lacking. Part e was rarely given credit, as many appeared to misread the question, and discussed the changes in first ionisation energies across Period 3.
There appeared to be some significant gaps in knowledge within this question, the various parts either scored very well or not at all.
In a(ii) there was a poor understanding of the nature of bonding in aluminium chloride and aluminium oxide. Candidates are still confusing electrical conductivity in compounds with that in metals, and often refer to the inability to conduct being down to a lack of mobile electrons in compounds.
Balancing equations, both full, as in parts a(i) and a(iii), and half equations as in b(ii), showed poor knowledge both of the reactants and products and in the ability to balance them in both atoms and charge. It should be expected that higher level candidates would be comfortable with these processes. The ability to deduce and predict what they would see during a reaction is a skill required of all chemists, it was missing in the attempts to answer b(ii). Parts c and d(i), ii) and iii) showed good knowledge, but in part d(iv) the understanding of the acid nature of some d block complex ions was lacking. Part e was rarely given credit, as many appeared to misread the question, and discussed the changes in first ionisation energies across Period 3.
There appeared to be some significant gaps in knowledge within this question, the various parts either scored very well or not at all.
In a(ii) there was a poor understanding of the nature of bonding in aluminium chloride and aluminium oxide. Candidates are still confusing electrical conductivity in compounds with that in metals, and often refer to the inability to conduct being down to a lack of mobile electrons in compounds.
Balancing equations, both full, as in parts a(i) and a(iii), and half equations as in b(ii), showed poor knowledge both of the reactants and products and in the ability to balance them in both atoms and charge. It should be expected that higher level candidates would be comfortable with these processes. The ability to deduce and predict what they would see during a reaction is a skill required of all chemists, it was missing in the attempts to answer b(ii). Parts c and d(i), ii) and iii) showed good knowledge, but in part d(iv) the understanding of the acid nature of some d block complex ions was lacking. Part e was rarely given credit, as many appeared to misread the question, and discussed the changes in first ionisation energies across Period 3.
There appeared to be some significant gaps in knowledge within this question, the various parts either scored very well or not at all.
In a(ii) there was a poor understanding of the nature of bonding in aluminium chloride and aluminium oxide. Candidates are still confusing electrical conductivity in compounds with that in metals, and often refer to the inability to conduct being down to a lack of mobile electrons in compounds.
Balancing equations, both full, as in parts a(i) and a(iii), and half equations as in b(ii), showed poor knowledge both of the reactants and products and in the ability to balance them in both atoms and charge. It should be expected that higher level candidates would be comfortable with these processes. The ability to deduce and predict what they would see during a reaction is a skill required of all chemists, it was missing in the attempts to answer b(ii). Parts c and d(i), ii) and iii) showed good knowledge, but in part d(iv) the understanding of the acid nature of some d block complex ions was lacking. Part e was rarely given credit, as many appeared to misread the question, and discussed the changes in first ionisation energies across Period 3.
There appeared to be some significant gaps in knowledge within this question, the various parts either scored very well or not at all.
In a(ii) there was a poor understanding of the nature of bonding in aluminium chloride and aluminium oxide. Candidates are still confusing electrical conductivity in compounds with that in metals, and often refer to the inability to conduct being down to a lack of mobile electrons in compounds.
Balancing equations, both full, as in parts a(i) and a(iii), and half equations as in b(ii), showed poor knowledge both of the reactants and products and in the ability to balance them in both atoms and charge. It should be expected that higher level candidates would be comfortable with these processes. The ability to deduce and predict what they would see during a reaction is a skill required of all chemists, it was missing in the attempts to answer b(ii). Parts c and d(i), ii) and iii) showed good knowledge, but in part d(iv) the understanding of the acid nature of some d block complex ions was lacking. Part e was rarely given credit, as many appeared to misread the question, and discussed the changes in first ionisation energies across Period 3.
There appeared to be some significant gaps in knowledge within this question, the various parts either scored very well or not at all.
In a(ii) there was a poor understanding of the nature of bonding in aluminium chloride and aluminium oxide. Candidates are still confusing electrical conductivity in compounds with that in metals, and often refer to the inability to conduct being down to a lack of mobile electrons in compounds.
Balancing equations, both full, as in parts a(i) and a(iii), and half equations as in b(ii) showed poor knowledge both of the reactants and products and in the ability to balance them in both atoms and charge. It should be expected that higher level candidates would be comfortable with these processes. The ability to deduce and predict what they would see during a reaction is a skill required of all chemists, it was missing in the attempts to answer b(ii). Parts c and d(i), ii) and iii) showed good knowledge, but in part d(iv) the understanding of the acid nature of some d block complex ions was lacking. Part e was rarely given credit, as many appeared to misread the question, and discussed the changes in first ionisation energies across Period 3.

